
Original Article
Austin J Vet Sci & Anim Husb. 2024; 11(1): 1138.
Prevalence of Schistosomiasis in Asymptomatic Dogs in Southeastern Louisiana, United States
Devora PA1; Bergeron C1,2; Minchew K1,3; Wolfson W1; Vatta AF4; Delcambre BA4; Liu C-C1; Moeller C1,5; Gaschen F1; Rademacher N1; Johnston AN1*
1Department of Veterinary Clinical Sciences, Louisiana State University School of Veterinary Medicine, USA
2St. Joseph’s Academy, Louisiana, USA
3Hammond Veterinary Services, Louisiana, USA
4Department of Pathobiological Sciences and Louisiana Animal Disease Diagnostic Laboratory, Louisiana State University School of Veterinary Medicine, USA
5Stanley S. Scott Cancer Center, Louisiana State University Health Sciences Center, USA
*Corresponding author: Johnston AN Department of Veterinary Clinical Sciences, Louisiana State University, School of Veterinary Medicine, Skip Bertman Drive, Baton Rouge, Louisiana 70803, USA. Tel: +1 225 578 9903 Email: johnston1@lsu.edu
Received: January 19, 2024 Accepted: February 27, 2024 Published: March 05, 2024
Abstract
The schistosome parasite, Heterobilharzia americana, infects various wild and domestic mammals in the southeastern and central United States, including the domestic dog. Dogs with schistosomiasis may be asymptomatic or have systemic illness due to granulomatous inflammation within the gastrointestinal tract, lungs, liver, lymph nodes, pancreas, and spleen. Diagnosis of schistosomiasis relies on non-routine diagnostic tests such as fecal saline sedimentation and targeted PCR amplification of H. americana DNA from fecal samples. The diagnosis may be supported by certain sonographic abnormalities, which have been shown to correlate with disease. We aimed to determine the prevalence of canine schistosomiasis in free-roaming and owned dogs in southeastern Louisiana. Thirty clinically healthy, adult, free-roaming dogs and 81 clinically healthy, adult, owned dogs were screened for H. americana infection. Positivity was defined by identification of H. americana DNA extracted from fecal samples and was confirmed by visualization of ova from fecal saline sedimentation via microscopy and Sanger sequencing of amplified DNA. Only one asymptomatic client owned dog (0.9%) was positive for H. americana. The infected dog was positive on both diagnostic laboratory tests and was also found to have an abnormal small intestinal submucosal layer and mesenteric lymph node enlargement on abdominal ultrasound, sonographic changes associated with H. americana infection.
Keywords: Canine; Fluke; Heterobilharzia americana; Schistosome
Abbreviations: cGAPDH: Canine Glyceraldehyde-3-Phosphate Dehydrogenase; CI: Confidence Interval; CT: Cycle Threshold; FSS: Fecal Saline Sedimentation; LSU: Louisiana State University; PCR: Polymerase Chain Reaction; RT-PCR: Real-Time Polymerase Chain Reaction
Introduction
Heterobilharzia americana, a parasitic schistosome, poses a health risk to dogs in the central and southeastern United States [1-3]. The natural definitive hosts of H. americana are the racoon (Procyon lotor) and nutria (Myocastor coypus), but domestic dogs (Canis familiaris) may also serve as a definitive host [1,4]. Dogs are infected when they come into contact with fresh water sources harboring lymnaeid snails, the intermediate host. Free swimming cercariae, released by the snails, penetrate the skin of the definitive host and migrate through the systemic vasculature to the lungs and liver where flukes mature. Heterobilharzia are dimorphic and reproduce sexually. After ova are fertilized, eggs are released into the mesenteric vasculature, where proteolytic enzymes released by the eggs enable migration through the intestinal wall and into the feces of the host. Ova migration promotes a pro-inflammatory immune response which exacerbates tissue injury. Granulomatous inflammation is evident histologically and can result in changes visible on ultrasonography. Ultrasonographic abnormalities correlated with canine schistosomiasis include splenomegaly, thickened gastric and small intestinal submucosa, enlarged mesenteric lymph nodes, and peritoneal effusion [5]. The most commonly associated small intestinal lesions were found to be heterogenous layering and a submucosal thickness exceeding 1.5mm [6]. Small intestinal submucosal thickening and the presence of pinpoint hyperechoic foci in the liver, small intestines, and mesenteric lymph nodes were found to be specific (96.4%) for H. americana infection in dogs [6]. Clinical signs of H. americana infection include anorexia, vomiting, diarrhea, and hematochezia, but remarkably some dogs are asymptomatic [4,7,8]. A retrospective study reviewing records of H. americana infection at the Texas Veterinary Medical Diagnostic Laboratory determined that H. americana was identified incidentally in 42% of samples from biopsy and necropsy submissions [4]. Of the 20 necropsy cases where death was attributable to H. americana infection, only one case was diagnosed ante mortem [4]. Veterinarians do not routinely screen for canine schistosomiasis, even in endemic regions. Noninvasive screening methods include Fecal Saline Sedimentation (FSS) or molecular diagnostics, however these methods are not commonly employed in dogs without clinical signs. It is plausible that canine schistosomiasis is more widespread than generally recognized. The primary objective of this study was to determine the prevalence of Heterobilharzia infection in owned and free roaming dogs in southeastern Louisiana.
Materials and Methods
Study Population
Thirty clinically healthy, free-roaming dogs (group 1) and 81 clinically healthy, owned dogs (group 2) were enrolled in the study. Adult dogs with normal stool from 2 shelters in southeastern Louisiana [1,2], were enrolled in group 1. Dogs owned by the students, faculty, and staff of the Louisiana State University (LSU) Veterinary Teaching Hospital and client owned dogs from a veterinary clinic in southeastern Louisiana were enrolled in group 2 if inclusion criteria were met. Dogs were included in the study if they were greater than 1 year of age, had no current or historic gastrointestinal signs (vomiting or diarrhea), and had not received praziquantel or fenbendazole within 3 months of the study. Patient data gathered about study subjects included age, sex, breed, and weight. Owner and shelter consent was collected prior to study participation. The study protocol was approved by the Louisiana State University School of Veterinary Medicine’s Institutional Animal Care and Use Committee (IAUCAM-21-056, approval date August 25, 2021; IACUCAM 23-020, approval date February 26, 2023).
Fecal Samples
Fecal samples were gathered between June 2021 and August 2023. Samples were stored at 4°C for a maximum of 3 days. Fecal samples were then stored at -20°C until DNA extraction and molecular testing was conducted.
Fecal Saline Sedimentation (FSS)
The positive sample was confirmed by Fecal Saline Sedimentation (FSS). Approximately 3 grams of feces were mixed with 15mL of 0.13M NaCl solution in a beaker to create a slurry. The slurry was processed using a system of differential filtration followed by differential sedimentation, the FLUKEFINDER (Soda Springs, ID). The strained slurry was poured into a 50mL conical tube and allowed to sediment for 5-10 minutes. The supernatant was decanted and 2mL of sediment was transferred to a nematode counting chamber (Nematode Counting Slide, Chalex Corp, Centreville, MD). The presence of H. americana ova was determined using a light microscope (Leitz SM-LUX microscope, Stuttgart, Germany) at 4x and 10x magnification. Images were obtained using a microscope camera attached to an Olympus BX43 microscope (Olympus SC180, Tokyo, Japan) at 10x and 40x magnification focusing on the plane corresponding to the upper surface of the lower slide of the counting chamber.
DNA Extraction
DNA was extracted from 1 gram of frozen feces using a commercial kit according to the manufacturer’s protocol (QIAamp Fast DNA Stool Mini Kit, QIAGEN, Valencia, CA) with modifications to increase DNA yield: fecal supernatant from centrifuged fecal lysate was increased from 200uL to 500uL. Nucleic acid concentration was determined using a spectrophotometer (Nanodrop 1000, Thermo Fisher Scientific, Waltham, MA).
Molecular Diagnostics
Real-time PCR (RT-PCR) was performed on 111 samples using TaqMan master mix (TaqMan® Universal PCR Master Mix, Applied Biosystems, Waltham, MA) as described [9]. Canine glyceraldehyde-3-phosphate dehydrogenase (cGAPDH) cDNA was amplified according to SYBR® manufacturer’s protocols (PerfeCTa® SYBR® Green FastMix® Quantabio, Beverly, MA) as a housekeeping control to test for PCR inhibitory substances. The average melting temperature and standard deviation for samples with mean cGAPDH Cycle Threshold (Ct) was determined. Samples with mean cGAPDH Ct values greater than 35 were not included in this study [10,11]. Primer and probe RT-PCR sequences are described (Table 1). Samples were run in duplicate with positive and negative controls using either 7900HT Applied Biosystems Real-Time PCR Detection System (Applied Biosystems, Waltham, MA) or QuantStudio 12K Flex Real-Time PCR system (Applied Biosystems, Waltham, MA). To confirm positivity, a unique DNA sequence corresponding to the 18S small subunit ribosomal RNA gene was amplified (DreamTaq® Green PCR Master Mix, ThermoFisher Scientific, Waltham, MA) and sequenced (Table 2). Sanger sequencing was performed by the LSU Gene Lab and nucleotide sequences were compared to NCBI accessions of the 18S ribosomal primer sequences: AY157220.1, HQ339878.1 [12].
Species
Direction
Sequence
Amplicon
Heterobilharzia americana
Primers [9]
F
5’-CTTTGCCTCCACTCTTCTTC-3’
95 bp
R
5’-GAAGATAAACCAGTGCAAGGATG-3’
Probe [9]
5’-/56-FAM/TGTGCTCAG/ ZEN/TTCCTCTTGTCGAG/3IABkFQ/-3’
Canis familiaris
GAPDH
F
5’-TCCCCACCCCCAATGTATC-3’
92 bp
R
5’-CAAGAAGGTAGTGAAGCAGGCA-3’
RT-PCR: Real-Time Polymerase Chain Reaction; F: Forward; R: Reverse; bp: Basepairs.
Table 1: Primer and probe sequences for RT-PCR.
Heterobilharzia americana
Direction
Sequence
Amplicon
PCR Primers [12]
F
5’-ATGGCCGTTCTTAGTTGGTG-3’
487 bp
R
5’-CGTCTTCTCAGCATCAGTCG-3’
Sequencing Primer
F
5’-TAATTCCGATAACGAACGAGACT-3’
PCR: Polymerase Chain Reaction; F: Forward; R: Reverse; bp: Base Pairs.
Table 2: Primers for PCR amplification and Sanger sequencing.
Diagnostic Imaging
A complete abdominal ultrasound was performed on the positive dog by a board-certified veterinary radiologist (NR), blinded to participant infection status. The dog was fasted for a minimum of 12 hours prior to imaging. Appropriate transducers for ultrasonography were chosen based on patient size to achieve optimal image quality. Images were obtained using a Phillips iU22 (Philips, Bothell, WA, USA) or Toshiba Aplio 300 (Toshiba, Tokyo, Japan) ultrasound machine. Ultrasonographic parameters (frequency, gain, focus) were adjusted for best quality image. The dog was restrained in lateral recumbency and the hair over the abdomen was clipped, as needed. Alcohol and coupling gel were applied to enhance contact.
Four criteria correlated to H. americana infection were assessed during abdominal ultrasound: small intestinal submucosal thickening, mesenteric lymph node size, the presence of nodules in the liver and hyperechoic foci within the liver, small intestines, or mesenteric lymph nodes.
Statistical Analysis
Statistical analysis was performed using a commercial software program (JMP? Statistical Discovery?, SAS Institute, Cary, NC) and 95% confidence intervals were calculated using score confidence intervals. Study subject demographics were evaluated using descriptive statistics.
Results
Study Population
Sex was not available for two dogs and intactness was not available for 6 dogs in group 1 (shelter). Thirteen (n=13/28; 46.4%) dogs in group 1 were male and 5 (n = 5/13; 38.5%) were castrated. Fifteen dogs in group 1 (n=15/28; 53.6%) were female and 5 (n=5/15; 33.3%) were spayed. Breed was recorded for 23 dogs.
Seventeen dogs were described as mixed breeds (n=17/23; 73.9%). Five dogs were described as American pit bull terriers (21.7%), and one (4.3%) as Australian shepherd. Ages reported for group 1 dogs were approximations, but all dogs were mature adults.
In group 2 (client owned), 40 dogs were male (n=40/81; 49.4%) and 36 were castrated males (n=36/40; 90%). Forty-one dogs were female (n=41/81; 50.6%) and 39 were spayed females (n=39/41; 95.1%). Multiple breeds were represented in this population with 44 classified as mixed breed dogs (n=44/81; 54.3%).
There were three (3.7%) of the following breeds in this study: Brittany spaniel, English springer spaniel, German shepherd, and miniature Schnauzer. There were two (2.4%) of the following breeds: Cairn terrier and Yorkshire terrier. One (1.2%) of each of the following breeds were included: American hairless terrier, Beagle, Bichon Frisé, Blue heeler, Border collie, Brussels griffon, Catahoula leopard dog, Dachshund, Dogo Argentino, English Mastiff, French bulldog, Golden retriever, Great Dane, Greyhound, Irish wolfhound, Labrador retriever, American pit bull terrier, Pomeranian, Rottweiler, Standard Poodle, and Shih Tzu. Age was available for 80 dogs. The median age of group 2 dogs was 5.75 years and ranged from 1 to 23 years of age.
Detection of H. Americana
One participant from group 2 was positive for H. americana on RT-PCR. Positivity was validated by FSS and Sanger sequencing (Figure 1). The other 110 dogs were negative for H. americana on RT-PCR resulting in an apparent prevalence of 0.9% (95% CI; [0.16, 4.93].
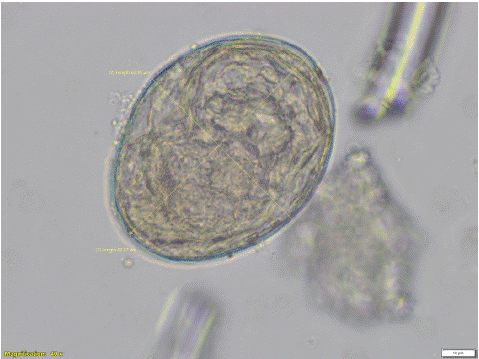
Figure 1: Heterobilharzia americana ovum collected from the stool of the positive dog (40x magnification). The egg width is 63.95 μm and the length is 82.27 μm.
Ultrasound Findings
A subjectively thickened small intestinal tunica submucosa measuring 1.33 mm was identified on the asymptomatic positive dog (Figure 2). Mesenteric lymph node enlargement (>8.2mm) was also reported in the dog with schistosomiasis. This finding corresponded with the description in [13]. Neither hypoechoichepatic nodules nor hyperechoic foci were not identified in the liver, small intestines, or spleen of the positive dog.
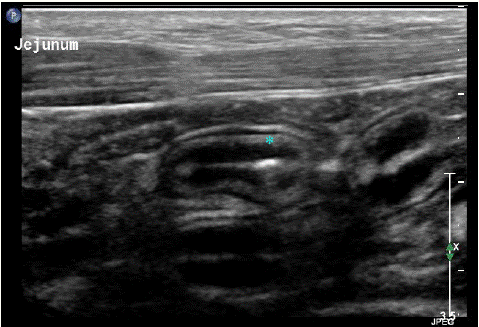
Figure 2: Ultrasound image of the small intestinal wall layering from the dog positive for Heterobilharzia americana. The asterisk indicates the small intestine tunica submucosa. The average small intestinal submucosal thickness was 1.33 mm (range: 1.2 – 1.5 mm).
Discussion
Only 1 of the 111 asymptomatic dogs screened was positive for H. americana, suggesting that the true prevalence in clinically healthy adult dogs is low in southeastern Louisiana. Furthermore, this rate is lower than that reported in a retrospective study in Texas, but similar to prospective and retrospective reports in Oklahoma, a recently indicated endemic region [4,14,15]. A definitive diagnosis of schistosomiasis can be established by identification of ova on fecal saline sedimentation, detection of H. americana DNA from canine fecal samples, or by identification of the eggs or flukes in histologic tissue sections. Although we relied on the more sensitive RT-PCR, it is possible that dogs with low fluke burden could have been missed. Further, in chronically infected patients the fecal shedding of ova reportedly reduces over time due to reduction in egg production by female worms [9]. This may have further confounded results.
One study reported that a combination of abnormal small intestinal wall layering and pinpoint hyperechoic foci in the small intestines, liver, or mesenteric lymph nodes were the most reliable indicators of canine H. americana infection (odds ratio: 36.87, positive predictive value: 94%, sensitivity: 58%) using abdominal ultrasonography, and 59.2% of infected dogs evaluated had submucosal thickening greater than 1.5mm [6]. The dog with asymptomatic schistosomiasis in our study had heterogeneous small intestinal wall layering with an average small intestine submucosal thickness of 1.33mm (range: 1.0-1.5mm) and mild mesenteric lymph node enlargement corresponding with previous reports, but lacked hyperechoic foci. The former changes may occur earlier than soft tissue mineralization resulting in hyperechoic foci.
Conclusion
This was the first study to prospectively evaluate healthy domestic dogs for H. americana infection. Only 1 of 111 dogs evaluated was infected with H. americana. As evident in the positive dog, sonographic changes associated with H. americana infection may also precede clinical signs. Ultrasonographic identification of an abnormal small intestinal submucosa should prompt screening for infection.
Author Statements
Authorship Contribution
Priscilla Devora: Methodology, Validation, Formal analysis, Investigation, Data curation, Writing- Original draft & review/editing, Visualization. Charley Bergeron: Investigation, Resources. Katelyn Minchew: Funding acquisition, Investigation. Cambri Moeller: Investigation. Wendy Wolfson: Resources, Supervision, Project administration, Funding acquisition. Frederic Gaschen: Supervision, Funding acquisition. Brooke Delcambre: Investigation, Resources, Supervision. Adriano Vatta: Investigation, Resources, Supervision. Chin-Chi Liu: Formal analysis, Data curation. Nathalie Rademacher: Conceptualization, Methodology, Investigation, Resources, Supervision, Project administration, Funding acquisition. Andrea Johnston: Conceptualization, Methodology, Investigation, Resources, Writing – Original draft & review/editing, Supervision, Project administration, Funding acquisition.
Conflict of Interest Statement
None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
Acknowledgments
We would like to acknowledge Pen Pals Animal Shelter and West Baton Rouge Animal Shelter for providing stool samples from free roaming dogs, as well as Dr. Adrian Bergeron from Highland Road Animal Hospital for providing stool samples from 51 owned dogs. The study was supported in part by the 2021 Comparative Gastroenterology Society / IDEXX Summer Scholar Award.
References
- Fabrick C, Bugbee A, Fosgate G, Clinical features and outcome of Heterobilharzia americana infection in dogs, J Vet Intern Med. 2010; 24: 140-4.
- Le Donne V, McGovern DA, Fletcher JM, Grasperge BJ, Cytologic Diagnosis of Heterobilharzia americana Infection in a Liver Aspirate From a Dog, Vet Pathol. 2016; 53: 633-6.
- Short RB, Grossman AI, Chromosomes of Heterobilharzia americana (Digenea: Schistosomatidae) from Texas, J Parasitol. 1986; 72: 807-9.
- Rodriguez JY, Lewis BC, Snowden KF, Distribution and characterization of Heterobilharzia americana in dogs in Texas, Vet Parasitol. 2014; 203: 35-42.
- Kvitko-White H, Sayre R, Corapi WV, Spaulding KA, Imaging Diagnosis - Heterobilharzia americana infection in a dog Vet Radiol Ultrasound. 2011; 52: 538-41.
- Moshnikova VS, Gilmour LJ, Cook AK, Fabiani M, Sonographic findings of pinpoint hyperechoic foci in the small intestine, liver, and mesenteric lymph nodes are indicative of canine Heterobilharzia americana infection, Vet Radiol Ultrasound. 2020; 61: 583-91.
- Graham AM, Davenport A, Moshnikova VS, Gilmour LJ, Fabiani M, Bishop MA, et al. Heterobilharzia americana infection in dogs: A retrospective study of 60 cases (2010-2019), J Vet Intern Med. 2021; 35: 1361-7.
- Hanzlicek AS, Harkin KR, Dryden MW, Chun R, Payne PA, Nietfeld JC, et al. Canine schistosomiasis in Kansas: five cases (2000-2009), J Am Anim Hosp Assoc. 2011; 47: e95-e102.
- Rodriguez JY. Heterobilharzia americana in dogs: Characterizing Clinical Infection, Evaluating diagnostic test performance, and exploring Novel Methods of Diagnosis [Dissertation]. Oaktrust Library: Texas A&M University. 2017.
- Goswami RS, Waldron L, Machado J, Cervigne NK, Xu W, Reis PP, et al. Optimization and analysis of a quantitative real-time PCR-based technique to determine microRNA expression in formalin-fixed paraffin-embedded samples, BMC Biotechnol. 2010; 10: 47.
- Oberli A, Popovici V, Delorenzi M, Baltzer A, Antonov J, Matthey S, et al., Expression profiling with RNA from formalin-fixed, paraffin-embedded material, BMC Med Genomics. 2008; 1: 9.
- Corapi WV, Ajithdoss DK, Snowden KF, Spaulding KA, Multi-organ involvement of Heterobilharzia americana infection in a dog presented for systemic mineralization, J Vet Diagn Invest. 2011; 23: 826-31.
- Agthe P, Caine AR, Posch B, Herrtage ME, Ultrasonographic appearance of jejunal lymph nodes in dogs without clinical signs of gastrointestinal disease, Vet Radiol Ultrasound. 2009; 50: 195-200.
- Duncan KT, Koons NR, Litherland MA, Little SE, Nagamori Y, Prevalence of intestinal parasites in fecal samples and estimation of parasite contamination from dog parks in central Oklahoma, Vet Parasitol Reg Stud Reports. 2020; 19: 100362.
- Nagamori Y, Payton ME, Looper E, Apple H, Johnson EM, Retrospective survey of endoparasitism identified in feces of client-owned dogs in North America from 2007 through 2018, Vet Parasitol. 2020; 282: 109137.