
Review Article
Thromb Haemost Res. 2023; 7(2): 1093.
“Increased Antithrombotic Properties of Unfractionated Heparin in Rats Following Oral Administration of Multiple Doses and Its Correlation with Endothelial Heparin Concentration”
Hari Prasad Sonwani*
Apollo College of Pharmacy, Durg CG, India
*Corresponding author: Hari Prasad Sonwani Apollo College of Pharmacy, Durg CG, India. Email: harisonwani10@gmail.com
Received: November 09, 2023 Accepted: December 23, 2023 Published: December 30, 2023
Abstract
In a rat model of venous thrombosis, a single oral dose of 7.5mg kg-1 of Unfractionated Heparin (UFH) lowers thrombosis by 50%. Since clinical long-term use is necessary, our goals were to investigate the antithrombotic effects of repeated oral UFH administration. Rats were given three doses of 7.5mg kg-1 every 12, 24, 48, and 72 hours apart—as well as three or fifteen doses of 1 mg kg—1 every 48 hours—by oral gavage of cow lung UFH. The final dosage was injecting 10% formalin in methanol into the jugular vein right after thrombus start. Four hours later, the vessel was checked for thrombosis. Heparin levels in endothelium and tissue as well as plasma anticoagulant activity were assessed. As 3×7.5 When heparin (mg kg-1) was administered, the incidence of thrombosis was notably lower during 48-hour dosing intervals than with a single dose. Endothelial heparin concentration and thrombotic incidence were negatively correlated, while anticoagulant activity was not. Treatment with three doses of 1mg kg-1 every 48 hours resulted in a thrombotic incidence comparable to that of a single dosage. The total thrombotic incidence after 15 doses was comparable to that following s.c. treatment and was lower than that of 3 doses. Oral UFH delivery resulted in increased antithrombotic activity and antithrombotic effects that were comparable to those of s.c. administration. Heparin’s antithrombotic effect on endothelium was linked to it.
Keyword: Heparin; Oral heparin; Venous thrombosis; Rats; Endothelium; Anticoagulant activity
Introduction
Heparins are essential medications that are used to stop blood clots in the veins [7]. Although lower molecular weight derivatives are injected Subcutaneously (s.c. ), Unfractionated Heparins (UFH) are provided via both intravenous and Subcutaneous (s.c.) routes. Heparin is thought to be inappropriate for absorption due to its large size and high negative charge, the low pH of the stomach, and the presence of digestive enzymes [1]. This view is also supported by the fact that there is little change in anticoagulant activity after oral treatment [8]. However, in rat venous and arterial thrombosis models, our research demonstrates that oral heparins prevent thrombosis [17,30]. The venous model showed a 50% decrease in the occurrence of stable thrombi following the gavage administration of 7.5mg-1 kg-1. Heparin may therefore be administered orally. Oral heparin would be especially helpful in situations when long-term administration is needed. It would save costs and make therapy more convenient for the patient by avoiding the hospital stay required for intravenous heparin. Additionally, it would stop haematomas and bruises that happen when s.c. administration is used. Additional heparin uses may also be possible with long-term oral heparin therapy. When administered orally, heparin has been shown to have the following positive effects: avoidance of vascular disease (Engelberg, adjuncts to chemotherapy drugs [26]. Few research have examined the consequences of oral heparin administered repeatedly without the use of delivery agents. Therefore, choosing the right dose for long-term therapy and considering the effects of repeated administration are crucial. This study sought to ascertain whether UFH accumulated in the body and whether it had an antithrombotic effect when administered repeatedly in a rat venous model. Additionally, we wanted to use a rat thrombosis model to determine the most efficacious repeated dosage schedule for oral heparin. Monitoring was also done on recovered heparin and plasma anticoagulant activity in tissues and endothelium.
Techniques
Heparin
Scientific Protein Labs, Division of Viobin Corporation, WI, USA provided the unfractionated bovine lung heparin (156. 2U mg-1), which was dissolved in water.
Animals at 3, 20, and 25 mg m. The Canadian Federation of Biological Societies' Principles of Animal Care were followed in the handling, housing, and conduct of all animal experiments. In total, 283 Wistar male rats, 305±4g (mean±standard error) in weight were acquired from Charles River in terms of the mean (s.e.m.) Prior to treatment, the animals were starved for a full night, and they were given barbital and methoxyflurane anesthesia for the experiments animal prototype. Four hours before the animals were killed; saline and the final dose of heparin were administered. When three repeating doses were administered daily, as well as during a 24-hour period before and on days 29–30, when fifteen repetitive doses were administered, rats were housed in metabolic cages with the purpose of collecting urine and faeces samples. Prior to heparin and on days 15 and 30, the animals were weighed.
Test for Thrombosis
A thrombus was started just before the final heparin dosage using a modified version of Blake et al.'s (1959) technique. The exposed jugular vein was treated with few drops of 10% formalin in 65% methanol, and the incision was then closed. The rat was anesthetized and checked for evidence of external bleeding after four hours. The wound was opened, and a cotton pledget was used to gently push the jugular vein to check for thrombi. If there was a visible thrombus in the jugular vein and there was restricted blood flow, the thrombus was graded as positive (stable thrombus). If the thrombus was visible in the jugular vein and moved when examined with a cotton pledget, the thrombus was considered unstable and the blood flow continued. If there was no visible thrombus and no disruption of blood flow, the thrombus was graded as negative. The same observer, who was not aware of the treatments, conducted the grading. By dividing the total number of events by the total number of thrombotic events, the percent incidence of both stable and unstable thrombi was determined.
Quantity of Animals Seen Receiving Therapy
Tissue collection As soon as the vessel was examined, tissue samples were taken. Samples of blood were drawn from the abdominal aorta in 3.8% sodium citrate (1 part sodium citrate to 9 parts blood). The inferior vena cava and thoracic aorta were excised and immersed in saline as a source of endothelium. Prairie Diagnostic Services, received a blood sample for complete blood counts. Next, plasma from blood samples taken with little tissue trauma was used to calculate the Activated Partial Thromboplastin Time (APTT). Extra aliquots of plasma were frozen in order to further determine the anti-Factor Xa or anti-Factor IIa activity. Lung, kidney, and liver were taken out.
After removal, the colon, ileum, jejunum, stomach, and duodenum were cleaned with distilled water. Frozen tissues and washes were used to extract heparin later on.
Heparin extraction and quantification from tissues and endothelium.
A modified version of a reported technique [15] was used to obtain endothelium. Vases were cut open, pinned lumen side up to dental wax, and then saline washed. Endothelium was removed from the lumenal surface after cellulose acetate membrane (Schleicher & Schuel Bio- Science, GmbH, Dassel, Germany) was applied.
Measuring to the nearest millimeter from the monolayer on cellulose acetate paper, the mean areas of the aorta and vena caval endothelium were 2.60±0.04 and 0.45±0.01 cm2 (mean±s.e.m.), respectively. The membrane made of cellulose acetate was taken from endothelium by centrifuging, removing the supernatant, and repeating the process twice in cold acetone. After the precipitates were allowed to air dry, 10 milliliters of pronase (derived from Streptomyces griseus, Sigma-Aldrich Canada) were added. 40mg kg-1 in 1 M Tris buffer) for 48 hours at 37 degrees Celsius (Limited, Oakville, ON, Canada). After centrifuging the digests for 10 minutes at 8000×g, the supernatant was collected and the precipitate washed twice. After washing 100 milliliters of 26.8% NaCl into the supernatant. Using five volumes of methanol and the supernatant, glycosaminoglycans were precipitated.
precipice was desiccated. Using modified techniques as previously described, heparin was recovered from tissue, gastrointestinal washes, and feces [23]. Following three consecutive dosages, saline washes were utilized to clean the gastrointestinal tissues, and the washes were collected. For the stomach, duodenum, jejunum, ileum, colon, liver, lung, and kidney, the average wet weights of tissue were 1.9, 1.8, 1.9, 1.8, 1.4, 9.1, 2.0, and 1.2 g, respectively. The average weights of the stomach, duodenum, jejunum, ileum, and colon washes were, respectively, 1.1, 0.4, 0.7, 0.8, and 2.7g. These weights were calculated by deducting the difference between the gut weights before and after washing. The total weight of excrement over a 24-hour period was 10.4g on average. Isoproterenol:petroleum ether and acetone were used to defat minced tissues, stomach washes, and excrement. (1:1), then processed at Dried precipitate was used.
According to previously published procedures, heparin was isolated from tissue, gut washes, and feces [23]. Saline was used to wash the gastrointestinal tissues after three consecutive dosages, and the washes were collected. The average tissue wet weights for the stomach, duodenum, jejunum, ileum, colon, liver, lung, and kidney were 1.9, 1.8, 1.9, 1.8, 1.4, 9.1, 2.0, and 1.2g, respectively. For stomach, duodenum, jejunum, ileum, and colon washes, the average weights were 1.1, 0.4, 0.7, 0.8, and 2.7g, respectively. These weights were calculated by deducting the difference in gut weight before and after washing. Over the course of a day, the weight of feces collected averaged 10.4g. Acetone and isoproterenol:petroleum ether were used to defat minced tissues, stomach washes, and feces and processed at (1:1).
At 405 nm, the sample's absorbance was measured. Heparin concentrations in anti-Factor Xa and anti-Factor IIa-based samples were determined by comparing the absorbance of the test samples to a standard curve that was created with known heparin addition quantities to control plasma. It was also tested using the APTT (Biopool, Ventura, CA, USA).
Data Evaluations
The mean±s.e.m. is used to express all results. Data on thrombosis are given in percentage form. The percentage of endothelial samples positive for heparin and thrombotic occurrences were compared using the w2-test for difference between proportions. Prior to analysis, the amounts of heparin in tissues and stomach washes were transformed using a logarithmic function to guarantee comparable variances among the groups. The average and as the antilog, s.e.m. are expressed; hence, positive and adverse are distinct and present in the tables and text. One-way analysis of variance and Tukey's multiple comparison test were used to analyze differences in endothelium and plasma heparin concentrations found by anticoagulant tests. When endothelium and plasma heparin concentrations were taken into account, a single dosage and 30-day controls were merged. A one-tailed t-test was used to compare variations in tissue heparin concentrations. The relationship between mean endothelium concentrations and the overall thrombotic incidence was examined using a Pearsons correlation. Po0.05 probability was regarded as substantial.
Outcomes
To find out if giving heparin orally in three consecutive doses as opposed to a single dose had an accumulative antithrombotic effect, preliminary research was conducted. The intervals between doses were adjusted to determine whether Antithrombotic activity was impacted by this. Furthermore, the effects of 15 doses of 1mg per kg administered by stomach tube, 3 doses of 1 mg per kg administered by oral gavage, and 15 doses of 1mg per kg administered by s.c. injection were compared to see if a cumulative effect could be observed after repeated dosing with oral heparin.
Antithrombogenic Properties
When compared to control rats, the incidence of stable thrombi was much lower after a single oral dose of 7.5mg kg-1 (Figure 1). The combined incidence of stable and unstable thrombi did not differ substantially from that of control rats.
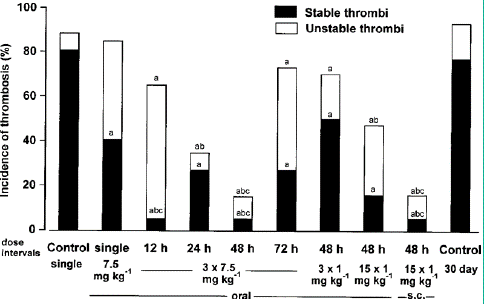
Figure 1: Antithrombotic effects in a rat jugular vein model when unfractionated heparin (UFH) was given by the oral route in repeated doses. UFH was given orally at 7.5 mg kg—1 as a single dose, three repeated doses 12, 24, 48 and 72h apart; or at 1 mg kg-1 in three repeated doses 48 h apart. In addition UFH was given orally or s.c. in 15 repeated doses at 1 mg kg-1, 48 h apart. Control rats were given oral saline as a single dose or for 30 days. The final dose was given immediately following thrombus initiation and vessels were examined 4h later. Significantly different from a, controls; b, single-dose UFH 7.5mg kg-1; c, 3×1mg kg-1 48h apart (w2-test for differences between proportions).
After receiving 7.5mg kg-1 for three consecutive Total thrombotic incidence was reduced with time between doses up to 48 hours for oral doses 12, 24, 48, and 72 hours apart. With dosing intervals of 12, 24, and 48 hours, but not 72 hours, the overall thrombotic incidence was considerably lower than following saline administration. With intervals of 24 and 48 hours, but not 12 and 72 hours, the total thrombotic incidence was considerably lower than for a single dose. Happenings at dosage intervals of 12, 24, 48, and 72 hours of steady thrombi were notably lower as compared to saline therapy.
Repeated dosage treatment significantly reduced the incidence of stable thrombi compared to single dose administration with dose intervals of 12 and 48 hours, but not of 24 and 72 hours.
Given that the incidence of stable thrombi was decreased with repeated dosing at 7.5mg kg-1, a lower dose (1 mg kg-1) was given as three doses at the ideal interval (48h), as shown by the data previously displayed in Figure 1. Compared to saline treatment, there was a significant decrease in the incidence of stable thrombi but not total thrombotic events (Figure 1).
When oral heparin was given in 15 doses as opposed to 3 doses of 1 mg per kg every 48 hours or with 30-day control rats, the incidence of stable thrombi was reduced (Figure 1). The incidence did not change between oral and s.c. treatment of stable thrombi. The 30-day controls' total thrombotic incidence was considerably higher than it was after 15 doses of 1 mg per kg every 48 hours, but not after three. Between oral and s.c. treatment, there was no discernible difference in the overall incidence of thrombosis. The total thrombotic incidence was considerably lower after 15 s.c., but not oral, doses of 1 mg per kg every 48 hours of UFH than it was after 3 doses of 1mg per kg every 48 hours.
Concentrations of Endothelials
After oral treatment, heparin was detected with both aorta and vena caval endothelium (Table 1). When three dosages of 7.5mg kg-1 were administered at 48 and 24 hours, the proportion of aortic endothelial samples positive for heparin was highest. h dosing intervals, which was notably higher than those of the controls or the 12- and 72-hour intervals. The amount that was extracted from the aorta endothelium was highest at 48-hour intervals, and it was considerably higher than that of the controls, 12 or 72-hour dosing intervals. The highest proportion of heparin-positive vena caval endothelial samples was observed at 48-hour intervals. Compared to controls, the percentage of positive samples increased considerably at 12, 24, and 48-hour intervals, but not at 72-hour intervals.
The amounts found on vena caval endothelium were greatest for 48 h followed by 24 intervals. Amounts found at 48 h intervals were significantly greater than controls or when heparin was given at 12 or 72h intervals.
There was a significant negative correlation (P=0.03).
between the mean amount of heparin found with vena caval endothelium and the total thrombotic incidence when rats were treated with three doses of 7.5mg kg-1 heparin (Figure 2). The negative correlation between mean amount of heparin found with aortic endothelium and the total thrombotic incidence approached significance (P=0.057).
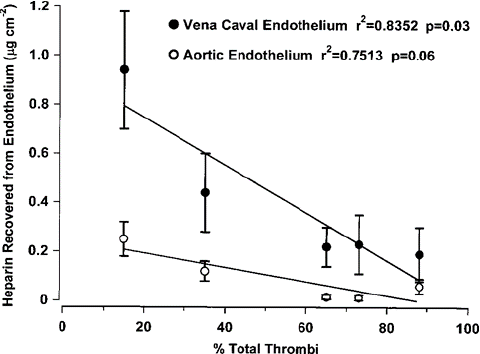
Figure 2: Correlation between antithrombotic activity and heparin recovered from vena caval and aortic endothelium following repeated dose administration. Heparin (7.5 mg kg-1) was adminis- tered in three repeated doses 12, 24, 48 or 72h apart immediately following thrombus initiation. Control rats were given saline. Thrombosis was scored and aorta and superior vena cava were harvested 4h after thrombus initiation. Mean±s.e.m. are shown for concentrations on aortic and vena caval endothelium. Pearson correlation calculation r2=0.8352, P=0.030 for vena cava; r2=0.7513, P=0.057 for aorta.
When heparin was given in three doses of 1 mg per kg per 48 h, incidence and amounts found on aortic or vena caval endothelium did not differ from controls. Heparin was also found with endothelium after 15 doses of 1mg per kg per 48h (Table 2). Per cent incidence of samples positive for heparin, for both oral and s.c. administration of 15 doses, was significantly greater than that of controls or after 3 doses of 1mg per kg per 48h. The amount found on vena caval endothelium after oral heparin was significantly greater than s.c. administration and in controls.
Plasma Anticoagulant Activity
Heparin administered in three doses of 7.5mg kg-1 in 12 and 24h intervals showed a small but significant increase in APTT versus controls, single-dose treatment and repetitive dosing at 48 and 72h (Table 3). There was no correlation between APTT and antithrombotic activity. There was no difference in heparin concentrations as determined by anti-Factor Xa activity between groups. Plasma heparin concentrations determined by anti-Factor Xa and IIa activity following 15×1mg per kg per 48h oral or s.c. heparin administration did not differ significantly from controls. A small but significant decrease in APTT was observed in rats receiving 15 doses of 1mg per kg per 48h when compared to control values or those given 3 doses of 1mg per kg per 48.
Distribution in Tissues
Heparin was found in all gut tissues and washes at all time intervals. When heparin was given at 3×7.5mg kg-1, most heparin was found in the stomach and stomach washes at 12 h intervals (Figure 3). The concentration of heparin in faeces increased as the intervals between administrations increased. Total amounts found in faeces were (mean±s.e.m. after log transformation; see Methods) 1.8(+0.7, -0.5), 12.2(+4.8, -3.5), 57.2(+12.5, -10.2), 327(+46.8, -54.6) micrograms after oral heparin given at 12, 24, 48 and 72h dose intervals, respectively. In the same animals, heparin was recovered from all non-gut tissue analysed. Heparin recovered from lung were 56.1(+59.7, -28.9), 3.4(+2.9, -1.4), 27.9(+55.1, +18.5) and 23.4(+23.2, -11.6) micrograms per gram of wet weight; from liver were 29.2(+39.2, -16.7), 2.2(+ 3.7, -1.4), 4.6(+ 3.5, -2.0) and 0.6(+1.3, -0.7) micrograms per gram of wet weight; and from kidney were 7.6(+3.9, -2.6), 0.6(+0.4, -0.2), 30.7(+4.7, -4.0) and 1.4(+1.2, -0.7) micrograms per gram of wet weight for 12, 24, 48 and 72h, respectively. Most heparin was recovered from lung and liver samples when given at 12h dose intervals. Total amounts recovered from urine were 0.86(+0.4, -0.3), 1.3(+0.7, -0.5), 3.1(+0.1, -0.7) and 0.6(+0.2, -0.7) microgram for 12, 24, 48 and 72h, respectively. There was no increase in amounts found in urine as intervals between dosing increased. When heparin was given as 15 doses of 1mg per kg per 48h over a 30-day period, only trace amounts were found in animal well-being.
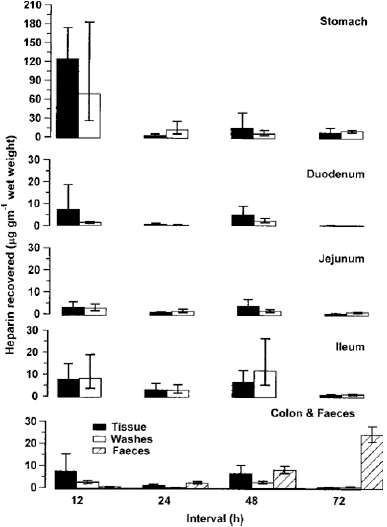
Figure 3: Heparin found in gut tissue, gut washes and faeces following oral administration of three repeated doses of 7.5mg kg-1 at different time intervals. Time intervals were 12, 24, 48 and 72h apart. Tissue and washes were harvested 4 h after the last dose from six rats per group selected at random. Mean±s.e.m. are shown. Faeces were collected from 20 rats per group throughout the time of heparin exposure.
Weight increase throughout the course of a 30-day period was 128.8±33.3, 144.5±28.9 g, and 145.0±17.8 g for oral, subcutaneous, and heparin administered in 15 doses of 1 mg per kg every 48 hours. Control treated, correspondingly, and showed no discernible variation between the groups. All haematological readings, including the leukocyte count, mean corpuscular volume, mean corpuscular hemoglobin concentration, and RBC count, were within normal ranges and there was no sign of bleeding. All haematological parameters were significantly different, with the exception of mean corpuscular volume. This was 54.8 fl in rats given oral heparin, which was comparable to those given s.c. heparin but significantly different from controls (57.33 fl). (55.0 fl) (one-way analysis of variance, P=0.047). Platetella count was typical despite indications of forty percent of the oral heparin-treated group had a few enlarged platelets.
Conversation
While oral heparin administration is thought to be inefficient, our lab and others have gathered a substantial amount of evidence in favor of the theory that heparin is absorbed without the need of delivery agents [14,9] (Hiebert, 2002). Certainfacts make oral heparin ineffective: it is thought that stomach acids break down heparin [28]; absorption is unlikely due to the drug's high molecular weight and negative charge [28]; and there is minimal change in anticoagulant activity after oral administration [8]. That being said, it is doubtful that stomach acidity breaks down heparin because heparin in 0.1 N HCl at 30 1C for 100 h showed no degradation, while in 0.1N HCl at 60 1C for 10h showed only 2% breakdown [22]. We and others observed small but significant changes in plasma antic- oagulant activity following oral administration to both rats and humans [18,20]. Engelberg showed a significant but small increase in APTT after giving oral heparin to 45 individuals [9]. Although changes in anticoagulant activity are minimal, considerable amounts of heparin are recovered from endothelium such that the endothelium can be considered as the prime distribution site (Hiebert, 2002). Heparin can also be recovered from urine in human studies [18]. We have also demonstrated that single doses of orally administered UFH and low molecular weight heparins have antithrombotic activity in rat venous (Hiebert, 2002) and arterial models [30], despite low plasma levels. Although, clinically, heparin is given in repeated doses, only a few studies have considered the effects of repeated dosing with orally administered heparin, without delivery agents. All report significant changes with evidence of absorption. A study in humans showed that oral heparin at a dose of 20 000 U twice weekly for 2–8 months showed slight prolongation of the APTT [9]. Unfrac-tionated or low molecular heparin at 0.5mg ml-1 in drinking water, given for 9–11 weeks to spontaneously hypertensive rats, returned systolic blood pressure to normal [33,34]. Oral heparin was also found to be effective in the survival of skin allografts and the treatment of rheumatoid arthritis (Gorski and Lagodzinski, 1991; Gorski et al., 1991) [14,21].
Our previous single-dose studies in a rat jugular vein model of venous thrombosis indicated that the dose required to reduce thrombosis by 50% was 7.5mg kg-1, while 3.25mg kg-1 had no antithrombotic effect [17]. In the present study, three oral doses of 7.5mg kg-1 of UFH, 24 or 48h apart, significantly reduced total thrombotic incidence, while doses given 12 and 48h apart, reduced the incidence of stable clots, compared to single-dose adminis- tration. This suggested that repetitive dosing was more effective than single-dose administration. This conclusion was supported by the observation that three doses of 1mg kg-1 of UFH, 48h apart, reduced thrombotic incidence by 50%, an effect similar to that observed after a single dose of 7.5mg kg-1, and was more effective than a single dose of 3.25mg kg-1 which was ineffective in previous studies [17]. Furthermore, 15 doses of 1mg per kg per 48h exerted an increased antithrombotic effect, compared to those after 3 doses. These observations clearly indicate accumulation of an antithrombotic effect following oral heparin administration.
Although we have previously observed heparin on endothelium after oral administration, this is the first time we have shown a clear negative correlation between thrombotic incidence and endothelial heparin. No such correlation was seen with anticoagulant activity, agreeing with previous observations indicating a poor correlation between APTT or anti-Factor Xa activity and antithrombotic activity [3,29].
This clearly indicates that heparin’s antithrombotic activity is more dependent on heparin on endothelium than plasma anticoagulant activity. The mechanism of accumulation of an antithrombotic effect is not entirely clear but may be related to increased heparin levels on endothelium. This is shown in the present study where more endothelial heparin was found when oral heparin was given for 15 doses of 1 mg per kg per 48 h versus 3 doses (Table 2). Furthermore, heparin can be found in all tissues investigated, indicating that it is widely distributed and thus may reflect attachment to, or accumulation within, endothelium. Considerable amounts of heparin were recovered from the lung, which may be due to the large vascular surface area in lung. In support, our previous studies found radioactivity in all tissues examined 24h following administration of 14C-labelled and -unlabelled porcine mucosal heparin, including gut tissue and washes and liver, lung, kidney, spleen, thymus, bone marrow, muscle, skin and so on [20]. In the present study, only small amounts were recovered from faeces and urine after repeated a dose, which agrees with previous observations where averages of 1.5 and 0.5% were found with urine and faeces, respectively, suggesting a slow excretion rate [20]. The present study also suggests that redistribution takes place. This was particularly evident in stomach tissue where more heparin was found after three doses of 7.5mg per kg per 12h than when the doses were given at longer intervals (Figure 3). The accumulation of effect was not related to anticoagulant activity in plasma.
There was little difference in antithrombotic activity when heparin was administered by the s.c. versus the oral route. This further supports the fact that orally administered UFH is absorbed. The effective dose of 1mg per kg per 48h is well within the dose recommended for s.c. administration to humans, initial dose 333U kg-1 followed by a fixed dose of 250U per kg per 12h [25].
The 15-dose regimen of orally administered heparin (by oral gavage) had no deleterious effect on the health of the animals. No bleeding was recorded and blood parameters were similar to controls. As thrombocytopenia is a side effect of heparin administration, platelets were monitored. Although enlarged platelets were observed, there was no change in platelet number. Failure to observe platelet changes could be due to species differences.
Thus studies on repetitive dosing further support the idea that orally administered UFH is absorbed and is an effective antithrombotic agent. Repeated dosing at 48 h intervals reduces the dose required compared with single dosing, indicating that accumulation occurs. Measurements of the amount recovered from tissue following repeated dosing suggests that heparin is only slowly excreted and that redistribution occurs. Antithrombotic activity was correlated with endothelial heparin but not anticoagulant activity. Repeated oral and s.c. heparin at the same dose had similar antithrombotic effects.
The used effective oral dose of 1 mg (150U) per kg per 48h is well within the range of effective clinical s.c. doses suggested for humans to prevent thrombosis and suggests an oral dosing regimen that should be tested further. Bleeding is not a side effect of oral UFH, but further studies are needed on the effect on platelets. Although orally administered UFH has an accumulative antithrombotic effect, further studies are needed to deter- mine whether accumulation occurs for other effects of oral UFH or for oral low molecular weight heparins.
References
- Arbit E, Goldberg M, Gomez-Orellana I, Majuru S. Oral heparin: status review. Thromb J. 2006; 4: 6.
- Bagasra O, Lischner HW. Activity of dextran sulfate and other polyanionic polysaccharides against human immunodeficiency virus. J Infect Dis. 1988; 158: 1084-7.
- Bara L, Planes A, Samama MM. Occurrence of thrombosis and haemorrhage, relationship with anti-Xa, anti-IIa activities, and D-dimer plasma levels in patients receiving low molecular weight heparin, enoxaparin or tinzaparin, to prevent deep vein throm- bosis after hip surgery. Br J Haematol. 1999; 104: 230-40.
- Berg D, Berg LH, Couvaras J, Harrison H. Chronic fatigue syndrome and/or fibromyalgia as a variation of antipho- spholipid antibody syndrome: an explanatory model and approach to laboratory diagnosis. Blood Coagul Fibrinolysis. 1999; 10: 435-8.
- Blake OR, Ashwin JG, Jaques LB. An assay for the antithrom- botic activity of anticoagulants. J Clin Pathol. 1959; 12: 118-22.
- Cornet AD, Smit EG, Beishuizen A, Groeneveld AB. The role of heparin and allied compounds in the treatment of sepsis. Thromb Haemost. 2007; 98: 579-86.
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007; 146: 278-88.
- Dryjski M, Schneider DE, Mojaverian P, Kuo BS, Bjornsson TD. Investigations on plasma activity of low molecular weight heparin after intravenous and oral administrations. Br J Clin Pharmacol. 1989; 28: 188-92.
- Engelberg H. Orally ingested heparin is absorbed in humans. Clin Appl Thromb Hemost. 1995; 1: 283-5.
- Engelberg H. Actions of heparin in the atherosclerotic process. Pharmacol Rev. 1996; 48: 327-52.
- Folkman J, Weisz PB, Joullié MM, Li WW, Ewing WR. Control of angiogenesis with synthetic heparin substitutes. Science. 1989; 243: 1490-3.
- Górski A, Lagodzinski Z. Oral heparin prolongs survival of skin allografts. Arch Immunol Ther Exp (Warsz). 1991; 39: 557-62.
- Górski A, Wasik M, Nowaczyk M, Korczak-kowalska G. Immunomodulating activity of heparin. FASEB J. 1991; 5: 2287-91.
- Gorski A, Imiela J, Nosarzewski J. Oral heparin in the treatment of rheumatoid-arthritis. J Immunol. 1993; 150: PA239.
- Hiebert LM, Jaques LB. The observation of heparin on endothelium after injection. Thromb Res. 1976; 8: 195-204.
- Hiebert LM, Ping T, Wice SM. Antithrombotic activity of orally administered low molecular weight heparin (logiparin) in a rat model. Haemostasis. 2000a; 30: 196-203.
- Hiebert LM, Wice SM, Jaques LB. Antithrombotic activity of oral unfractionated heparin. J Cardiovasc Pharmacol. 1996; 28: 26-9.
- Hiebert LM, Wice SM, Ping T. Increased plasma anti-Xa activity and recovery of heparin from urine suggest absorption of orally administered unfractionated heparin in human subjects. J Lab Clin Med. 2005; 145: 151-5.
- Hiebert LM, Wice SM, Ping T, Herr D, Laux V. Antithrombotic efficacy in a rat model of the low molecular weight heparin, reviparin sodium, administered by the oral route. Thromb Haemost. 2001; 85: 114-8.
- Hiebert LM, Wice SM, Ping T, Hileman RE, Capila I, Linhardt RJ. Tissue distribution and antithrombotic activity of unlabeled or 14C-labeled porcine intestinal mucosal heparin following administration to rats by the oral route. Can J Physiol Pharmacol. 2000b; 78: 307-20.
- Imiela J, Nosarzewski J, Górski A. Oral heparins in the treatment of rheumatoid arthritis. Arch Immunol Ther Exp (Warsz). 1995; 43: 313-5.
- Jandik KA, Kruep D, Cartier M, Linhardt RJ. Accelerated stability studies of heparin. J Pharm Sci. 1996; 85: 45-51.
- Jaques LB. Determination of heparin and related sulfated mucopolysaccharides. Methods Biochem Anal. 1977; 24: 203-312.
- Jaques LB, Wice SM, Hiebert LM. Determination of absolute amounts of heparin and of dextran sulfate in plasma in microgram quantities. J Lab Clin Med. 1990; 115: 422-32.
- Kearon C, Ginsberg JS, Julian JA, Douketis J, Solymoss S, Ockelford P, et al. Comparison of fixed-dose weight-adjusted unfractio- nated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. JAMA. 2006; 296: 935-42.
- Levine MN, Lee AY, Kakkar AK. From Trossseau to targeted therapy:new insights and innovations in thrombosis and cancer. J Thromb Haemost. 2003; 1: 1456-63.
- Mandal AK, Lyden TW, Saklayen MG. Heparin lowers blood pressure:biological and clinical perspectives. Kidney Int. 1995; 47: 1017-22.
- Money SR, York JW. Development of oral heparin therapy for prophylaxis and treatment of deep venous thrombosis. Cardiovasc Surg. 2001; 9: 211-8.
- Morris TA. Heparin and low molecular weight heparin: background and pharmacology. Clin Chest Med. 2003; 24: 39-47.
- Pinel C, Wice SM, Hiebert LM. Orally administered heparins prevent arterial thrombosis in a rat model. Thromb Haemost. 2004; 91: 919-26.
- Prajapati DN, Newcomer JR, Emmons J, Abu-Hajir M, Binion DG. Successful treatment of an acute flare of steroid-resistant Crohn’s colitis during pregnancy with unfractionated heparin. Inflamm Bowel Dis. 2002; 8: 192-5.
- Vasdev S, Ford CA, Longerich L, Barrett B, Parai S, Campbell N. Oral treatment with low molecular weight heparin normalizes blood pressure in hypertensive rats. Artery. 1994; 21: 1-28.
- Vasdev S, Sampson CA, Longerich L, Prabhakaran VM, Parai S. Oral heparin normalizes blood pressure and elevated cytosolic calcium in hypertensive rats. Artery. 1992; 19: 124-46.