
Special Article - Red Blood Cells
Thromb Haemost Res. 2019; 3(2): 1024.
Potential Diagnostic Application of the Quantitative Assessment of Red Blood Cell Membrane Protein Expression
Várady Gy1, Szabó E1,2, Kulin A1, Zámbó B1, Mózner O1, Pálinkás M3, Poór Gy3 and Sarkadi B1,4*
1Institute of Enzymology, Research Centre for Natural Sciences, Hungarian Academy of Sciences, Magyar Tudósok krt. 2, Budapest, Hungary, 1117
2Biospirál-2006 Kft., Temesvári krt 62. Szeged, Hungary, 6726
3National Institute of Rheumatology and Physiotherapy, Frankel Leó u. 25-29. Budapest, Hungary,1023
4Department of Biophysics and Radiation Biology, Semmelweis University, Tuzoltó u. 37-47, Budapest, Hungary, 1094
*Corresponding author: Sarkadi B, Institute of Enzymology, Research Centre for Natural Sciences, Hungarian Academy of Sciences, Magyar Tudósok krt. 2, Budapest, Hungary; Email: sarkadi@biomembrane.hu
Received: May 06, 2019; Accepted: May 31, 2019; Published: June 07, 2019
Abstract
We have recently developed an efficient flow cytometry-based method which allows to quantitatively determine the expression levels of selected Red Blood Cell (RBC) membrane proteins. Interestingly, RBCs express several hundred membrane proteins, the expression of which is determined by the individual genetic background and complex regulatory effects. Many of these membrane proteins are closely linked to the development of human diseases or diseasesusceptibilities, thus the determination of specific RBC protein expression levels may help to perform a patient-specific, stratified diagnosis. In this technology we generate RBC ghosts with a simple fixation procedure, then use monoclonal antibodies optimized to recognize specific RBC membrane proteins. The data obtained in selected groups of patients and control subjects are compared, and the genetic basis of the alterations in membrane protein expression is further examined by performing specific SNP and mutation analysis from genomic DNA samples. In this mini-review we summarize our recent studies related to changes in RBC protein expression in gout patients, and describe an interesting discovery based on the RBC expression studies of a plasma membrane calcium pump.
Keywords: Red blood cells; Flow cytometry; ABCG2; Gout; PMCA; Malaria; Biomarkers
Abbreviations
FACS: Fluorescence-Activated Cell Sorter; GWAS: Genome- Wide Association Studies; RBC: Red Blood Cell; SNP: Single Nucleotide Polymorphism; WGA: Wheat Germ Agglutinin
Introduction
Methodology for assessing RBC membrane protein expression and its medical role
Relevant RBC membrane proteins are selected based on the literature (red blood cell CD antibodies, GWA and medical diagnostic studies), and these data are compared to Mass Spectrometry (MS) measurements and the data in the erythrocyte database [1-3]. For Flow Cytometry (FACS) measurements, RBC ghosts (hemoglobin depleted erythrocyte membranes) are prepared from a small drop of blood and these ghosts are labeled by a fluorescent lectin derivative (e.g. wheat germ agglutinin-AlexaFluor647, WGA-A647) to allow selective gating. Thereafter, the ghosts are labeled with specific monoclonal antibodies, then with suitable fluorescent secondary antibodies (for details see [3,4]). The measurements are carried out in a flow cytometer, preferably equipped with automatic high throughput plate sampler.
In order to obtain quantitative results in the FACS assay, each membrane protein and each selective first antibody has to be carefully titrated to give maximum level interactions. Still, the results of the FACS measurements do not provide an actual number of the given membrane protein per RBC, and data have to be evaluated based on the protein levels obtained in a certain population or under specific disease conditions.
The advantage of the FACS assay lies in the quick and cost-efficient method that requires only a small amount of blood. However, a major issue in this work is the selection of proper antibodies for trustworthy, quantitative membrane protein measurements. In most cases no information is available about the use of commercially available antibodies in flow cytometry, and the quantities of most of the red blood cell membrane proteins are also unknown. In this latter regard, recently improving quantitative mass spectrometry measurements may provide proper estimates [1,2,4]. Still, for the quantitative detection of a particular RBC membrane protein, the use of at least two different antibodies, favorably recognizing different epitopes of the same protein, are preferred. In our research projects we have examined numerous commercially available monoclonal antibodies, potentially suitable for the characterization of RBC membrane proteins that is showing specific and sensitive detection of membrane protein levels even at low protein concentrations (Table 1).
Protein
Gene
Molecular function
Biological function
ABCA1
ABCA1
ATP binding cassette transporter, cholesterol / lipid transport
role in cellular efflux of cholesterol and lipids
ABCG2
ABCG2
ATP binding cassette transporter, xenobiotic and drug transport, urate transporter
role in drug ADME-tox, urate metabolism, cancer drug resistance
GLUT-1
SLC2A1
glucose transmembrane transporter activity in many cell types
glucose uptake, role in ascorbic acid metabolism
GLUT-3
SLC2A3
glucose transmembrane transporter activity mainly in neurons
glucose uptake, role in ascorbic acid metabolism
GLUT-4
SLC2A4
glucose transmembrane transporter activity in many cell types, mainly in adipocytes
glucose uptake
IR
INSR
insulin receptor, insulin binding, protein tyrosine kinase activity
glucose homeostasis
PMCA4b
ATP2B4
transmembrane calcium transport and ATPase activity, main Ca2+ exporter in many tissues
role in Ca2+ dependent signal transduction
SGLT2
SLC5A2
Sodium/glucose symporter activity
role in carbohydrate metabolic processes
URAT-1
SLC22A12
urate/anion antiporter
role in urate re-absorption in the kidney, regulates blood urate levels, response to drugs
Table 1: Examples of membrane proteins with medical relevance and with established antibody-based quantitative assays in RBCs.
Parallel with the FACS assays, genomic DNA is prepared from the same small blood samples, in order to study relevant individual polymorphisms and/or mutations. The alterations in the membrane protein levels found in individual samples thus can be further examined by genetic testing. As a first approach, disease-related Single Nucleotide Polymorphisms (SNPs) in the genes of the selected membrane proteins are searched for in related GWA studies, and the identified, sufficiently frequent variants are tested by PCR, e.g. using TaqMan assays. In cases of individuals showing a large difference in a given RBC protein level from the mean level in the population, the relevant genes are sequenced to search for potential rare mutations. In these cases, both the variants in the coding sequences and/or in the promoter/enhancer regions may give relevant information about the source of protein expression differences. Missense variations in the coding regions may cause major folding or trafficking problems, while mutations in the promoter/enchancer regions may significantly affect membrane protein expression levels through regulatory pathways [5-7].
Based on these experiments, the main goal is to connect the red blood cell membrane protein alterations with disease susceptibility, drug toxicity, treatment sensitivity or other relevant medical variables. The connection between the SNPs, the membrane protein levels, and the medical variables has to be statistically analyzed to uncover important correlations, providing a diagnostic value. The aim of our recent studies described below as examples, has been to uncover connections between RBC membrane protein changes and related genetic variations, which may play a role in the development or treatment possibility of diseases.
Selected RBC membrane protein studies with potential diagnostic applications
A. Gout and the expression of the ABCG2 protein in RBCs: Gout is a painful disorder of purine metabolism, with deposition of urate crystals in intra-articular and peri-articular areas. The basis of this deposition is an increased uric acid concentration in the blood, either because of a hyperproduction, or a reduced excretion of this physiological metabolite. The major site of the uric acid secretion is the kidney, but extrarenal secretion, especially in the intestine, is also important [8]. Urate deposition in the joints result in inflammation, episodic gout flames, gouty arthropathy, tophi formation, and often appears together with urolithiasis [9,10].
The ABCG2 protein is a multidrug transporter, which plays an important physiological role in the transport of xeno-and endobiotics and, when overexpressed, may cause multidrug resistance in cancer. This protein is mostly expressed in barrier tissues (e.g. the gut and the blood-brain barrier), in the liver and the kidney, and significantly modulates drug absorption, distribution, excretion and toxicity (ADME-Tox) properties [11]. Interestingly, it has been revealed, that ABCG2 is also an important transporter for uric acid excretion, especially in the intestine [12]. A relatively frequent ABCG2 polymorphism, Q141K (C421A), resulting in reduced protein expression and function, has been found in GWA studies as a major factor in gout formation [13].
It has been relatively recently (in 2012) discovered that ABCG2 is expressed in the red cell membrane, and the lack of this expression results in a rare blood group variant, J- [14]. In our related experiments we have analyzed the expression of this protein by our flow cytometry methods, coupled with a genetic test. This study [5]. showed that the ABCG2 protein can be quantitatively measured in the RBC membrane, the Q141K polymorphism in a heterozygous form results in a decreased RBC-ABCG2 level, and an even lower protein expression is seen in homozygous ABCG2-Q141K individuals, or in individuals (and their relatives) carrying a stop or a frameshift mutation of ABCG2 in a heterozygous form. In a further work [6], by using the same approach and analyzing individual samples with lower expression of ABCG2 in the RBC membrane, we found a relatively frequent new mutant variant, ABCG2-M71V, which also has a major expression and trafficking problem.
Recently we have initiated a study to investigate the correlation between lower RBC membrane ABCG2 expression and gout formation. In these, as yet unpublished experiments, we found a correlation between the RBC membrane expression levels of the ABCG2 and its variants and the appearance of gout at the clinic (Figures 1 and 2). In the gout patients we also found several less frequent mutations, which probably also contribute to the lower ABCG2 expression levels. Thus, lower RBC expression of the ABCG2 protein may guide further diagnostic and therapeutic approaches in these patients.
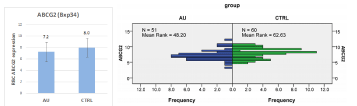
Figure 1: Left panel: average ABCG2 levels in the healthy and gout patients, respectively. The right panel shows the distribution frequency of the ABCG2 levels in
the two groups. AU: gout patients, CTRL: control group.
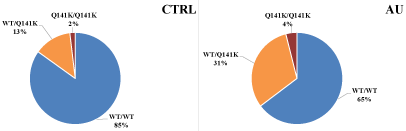
Figure 2: Differences between the frequencies of the Q141K polymorphism in healthy control (CTRL) individuals and in the gout patients (AU).
B. Expression of the calcium pump protein, PMCA4b in RBC membranes - a potential role in malaria susceptibility: The Plasma Membrane Ca2+-ATPases (PMCAs) are important active calcium exporter proteins in the human cells. The main function of these proteins is to maintain cellular calcium homeostasis by ensuring the low concentration of cytoplasmic calcium and participating in calcium dependent signal transduction. The PMCA proteins in human have four major isoforms, coded by four different genes. In addition, they have numerous tissue-specific splice variants, and the human red blood cells mostly express the PMCA4b variant [15-18].
When measuring the expression levels of PMCA4b in the RBC with a monoclonal antibody, we found several individuals with much lower expression levels than the mean value in the population. As measured in independent calcium transport experiments, the RBCs of individuals with lower PMCA4 expression had a much lower calcium extrusion capacity, thus reduced PMCA expression correlated with reduced transport function [7].
In order to find out the potential genetic background of the lower protein expression, we sequenced in these individuals the exons of the ATP2B4 gene coding for the PMCA4b protein. Interestingly, we did not find any mutations in the coding sequences of any of the samples. To further explore the potential genetic background of the reduced protein expression, we determined numerous SNPs in the potential promoter and/or enhancer regions of the PMCA4 gene. As shown in Figure 3, we found that the SNPs characteristic for a minor haplotype around exon 2 (Haplotype 1, which includes a potential alternative promoter in erythroid cell lines [19], an enhancer, and the translational start site), correlated with the reduced PMCA4b expression level. No other haplotypes or SNPs in the gene showed such a correlation with membrane protein expression levels.
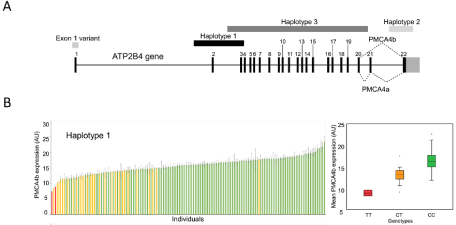
Figure 3: PMCA expression level correlation with genetic analysis. A, Important frequent SNPs and haplotypes of the ATP2B4 gene. B, The relationship of protein
expression and the SNPs of the haplotype of ATP2B4 gene. Minor allele homozygotes: red, minor allele heterozygotes: yellow, major alleles: green. Right side:
Mean values +/- SD values (at least two independent parallels), of the PMCA4b expression levels versus the ATP2B4 genotypes (Kruskal-Wallis tests). Modified
from Zámbó et al. [7].
It has been also suggested, that this region can be a potential alternative promoter in erythroid cells [19]. A recent study, analyzing the potential enhancer of the PMCA4 gene, found an erythroidspecific small enhancer region, corresponding to an area within Haplotype 1 [20].
Most interestingly, in a GWA study, the SNPs in Haplotype 1 have been found to correlate with a protection against malaria [21]. Moreover, based on CDC and NCBI data, this Haplotype occurs at much higher frequency in malaria infected regions – see Figure 4. These data suggest that malaria infection susceptibility is much lower in individuals with lower RBC PMCA4b protein, and this expression difference is a selection force in the major malaria-infected regions.
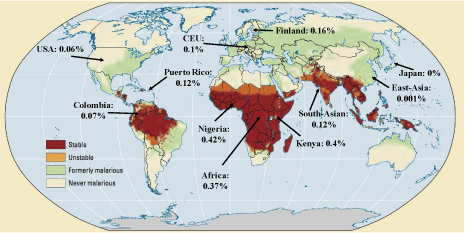
Figure 4: World map of past and current malaria prevalence with MAF% of SNPs in haplotype 1. Red: stable malaria infection, orange: periodic malaria infection,
green: previously infection, white: free from infection. Modified from: https://www.reddit.com/r/MapPorn/comments/65zpt5/os_malaria_past_and_current_
prevalence_all_around/?utm_source=share&utm_medium=web2x
The potential mechanism behind this effect is that Plasmodium proliferates in the so called parasitophore vacuole within the red blood cells, and Gazarini et al. [22], suggested that the parasite may use the PMCA4b of the inverted human erythrocyte membrane to maintain elevated calcium levels within this vacuole. Therefore, an active calcium RBC pump is required to allow parasite survival and growth, and a reduced PMCA4b expression in the erythrocytes may decrease the survival of this infectious agent.
Conclusion
A large number of membrane protein alterations are closely linked to the development of various human diseases, while the quantitative estimation of these proteins in the human tissues is practically unavailable. Interestingly, the determination of membrane protein expression levels in the RBCs in many cases reflects overall genetic or regulatory alterations, and may help to perform a patientspecific, stratified diagnosis. There could be several limitations in this approach, as tissue-specific regulation of membrane protein expression may not appear in the RBC samples. Still, our approach may help personalized medical diagnostics, and we are currently involved in clinically oriented RBC protein expression studies in major metabolic diseases, including gout and diabetes. The results of the combined flow cytometry and molecular genetic assay platform presented here may efficiently help to explore further diagnostic and therapeutic possibilities in these multifactorial diseases.
Acknowledgement
This work has been supported by a research grant from OTKA_K 115375 to Sarkadi B, OTKA_K 128011 to Várady Gy, and FIEK_16- 1-2016-0005.
References
- Hegedus T, Chaubey PM, Varady G, Szabo E, Saranko H, Hofstetter L, et al. Inconsistencies in the red blood cell membrane proteome analysis: generation of a database for research and diagnostic applications. Database. 2015; 1–8.
- Bryk AH, Wisniewski JR. Quantitative Analysis of Human Red Blood Cell Proteome. J Proteome Res. 2017; 16: 2752–2761.
- Várady G, Cserepes J, Németh A, Szabó E, Sarkadi B. Cell surface membrane proteins as personalized biomarkers: where we stand and where we are headed. Biomark Med. 2013; 7: 803–819.
- Várady G, Szabó E, Fehér á, Németh A, Zámbó B, Pákáski M, et al. Alterations of membrane protein expression in red blood cells of Alzheimer’s disease patients. Alzheimer’s Dement Diagnosis, Assess Dis Monit. 2015; 1: 334–338.
- Kasza I, Várady G, Andrikovics H, Koszarska M, Tordai A, Scheffer GL, et al. Expression Levels of the ABCG2 Multidrug Transporter in Human Erythrocytes Correspond to Pharmacologically Relevant Genetic Variations. PLoS One. 2012; 7: e48423.
- Zámbó B, Bartos Z, Mózner O, Szabó E, Várady G, Poór G, et al. Clinically relevant mutations in the ABCG2 transporter uncovered by genetic analysis linked to erythrocyte membrane protein expression. Sci Rep. 2018; 8: 7487.
- Zámbó B, Várady G, Padányi R, Szabó E, Németh A, Langó T, et al. Decreased calcium pump expression in human erythrocytes is connected to a minor haplotype in the ATP2B4 gene. Cell Calcium. 2017; 65: 73–79.
- Bobulescu IA, Moe OW. Renal transport of uric acid: evolving concepts and uncertainties. Adv Chronic Kidney Dis. 2012; 19: 358–371.
- Lee YH. Gout and the risk of Alzheimer's disease: A Mendelian randomization study. Int J Rheum Dis. 2019.
- Stamp LK, Chapman PT. Gout. In: Comorbidity in Rheumatic Diseases. Cham: Springer International Publishing. 2017; 179–195.
- Szakács G, Váradi A, Özvegy-Laczka C, Sarkadi B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME–Tox). Drug Discov Today. 2008; 13: 379-393.
- Stiburkova B, Pavelcova K, Zavada J, Petru L, Simek P, Cepek P, et al. Functional non-synonymous variants of ABCG2 and gout risk. Rheumatology. 2017; 56: 1982–1992.
- Nakayama A, Nakaoka H, Yamamoto K, Sakiyama M, Shaukat A, Toyoda Y, et al. GWAS of clinically defined gout and subtypes identifies multiple susceptibility loci that include urate transporter genes. Ann Rheum Dis. 2017; 76: 869-877.
- Saison C, Helias V, Ballif BA, Peyrard T, Puy H, Miyazaki T, et al. Null alleles of ABCG2 encoding the breast cancer resistance protein define the new blood group system Junior. Nat Genet. 2012; 44: 174–177.
- Caride AJ, Filoteo AG, Enyedi A, Verma AK, Penniston JT. Detection of isoform 4 of the plasma membrane calcium pump in human tissues by using isoform-specific monoclonal antibodies. Biochem J. 1996; 316: 353–359.
- Borke JL, Minami J, Verma A, Penniston JT, Kumar R. Monoclonal antibodies to human erythrocyte membrane Ca++-Mg++ adenosine triphosphatase pump recognize an epitope in the basolateral membrane of human kidney distal tubule cells. J Clin Invest. 1987; 80: 1225–1231.
- Bogdanova A, Makhro A, Wang J, Lipp P, Kaestner L. Calcium in Red Blood Cells—A Perilous Balance. Int J Mol Sci. 2013; 14: 9848–9872.
- Strehler EE. The ATP2B Plasma Membrane Ca2+ ATPase Family: Regulation in Response to Changing Demands of Cellular Calcium Transport. In: Chakraborti S, Dhalla NS, editors. Regulation of Ca2+-ATPases, V-ATPases and F-ATPases. 2016; 63–80.
- Band G, Le QS, Clarke GM, Kivinen K, Hubbart C, Jeffreys AE, et al. New insights into malaria susceptibility from the genomes of 17,000 individuals from Africa, Asia, and Oceania. bioRxiv. 2019.
- Lessard S, Gatof ES, Beaudoin M, Schupp PG, Sher F, Ali A, et al. An erythroid-specific ATP2B4 enhancer mediates red blood cell hydration and malaria susceptibility. J Clin Invest. 2017; 127: 3065–3074.
- Timmann C, Thye T, Vens M, Evans J, May J, Ehmen C, et al. Genomewide association study indicates two novel resistance loci for severe malaria. Nature. 2012; 489: 443–446.
- Gazarini ML, Thomas AP, Pozzan T, Garcia CRS. Calcium signaling in a low calcium environment. J Cell Biol. 2003; 161: 103–110.