
Research Article
Adv Res Text Eng. 2024; 9(2): 1097.
Study the Lawsone Dye Extract with Different Solvents Influences the Surface of Polyethylene Terephthalate Fabric
Sliman Hashim1,2,3; Xue D1,2; Xiaoye B1,2; Tao Zhao1,2*
1Department of Textile Chemistry and Dyeing and Finishing Engineering, College of Chemistry and Chemical Engineering and Biotechnology, Donghua University, Shanghai 201620, China
2Key Lab of Science and Technology of Eco-Textiles, Donghua University, Ministry of Education, Shanghai 201620, China
3Department of Textile, Industrial Research Consultancy Center, Ministry of Industry, Khartoum, Sudan
*Corresponding author: Tao Zhao Department of Textile Chemistry and Dyeing and Finishing Engineering, College of Chemistry and Chemical Engineering and Biotechnology, Donghua University, Shanghai 201620, China;
Key Lab of Science and Technology of Eco-Textiles, Donghua University, Ministry of Education, Shanghai 201620, China. Tel: +86-21-67792811; Fax: +86-21-67792811 Email: tzhao@dhu.edu.cn
Received: January 26, 2024 Accepted: March 04, 2024 Published: March 11, 2024
Abstract
Polyethylene Terephthalate (PET) fabric typically has a crystalline structure and hydrophobic properties, results in low moisture absorption, and generates anti-static properties. In this work, Lawsone Dye Extract (LDE) was extracted in different solvents to study the influence of Lawsone hybridization on the surface of PET fabric. During the dyeing process, the IR dyeing machine accelerates the dyeing process and speeds up the chemical reactions between the Lawsone dye and the PET fabric. Increased efficiency mainly allows them to diffuse more easily into the fabric’s fibers. This enhanced penetration results in improved dye uptake and potentially deeper coloration, leading to a more vibrant and uniform appearance of the dyed fabric. The surface energy of PET fabric increased; these interactions are a form of intermolecular force that affects an increase in surface energy. We used FTIR, XPS, XRD, and UV-VIS to investigate the surface morphology and chemical composition. However, Lawsone contains hydrophilic functional groups, such as hydroxyl groups (-OH), that can enhance the fabric’s affinity for water, confirmed by decreasing the Water Contact Angle (WCA) to zero. Accordingly, electrostatic charge records an improvement. Furthermore, both solvents produce durable colours in various intensities due to hybridization, which can be accurately evaluated by comparing the K/S value with their wash fastness. Overall, this technique offers a combination of sustainability and comfortable apparel. These findings drew an insightful understanding of natural dyes with PET surface, providing a comprehensive investigation of surface energy.
Keywords: Polyethylene Terephthalate (PET); Lawsone, Hybridization; Hydrophilicity; Anti-static
Introduction
Further research is necessary to study the chemical mechanisms and hybridization effects of natural dyes on PET fabric during dyeing [1-3]. Natural dyes possess colour since they contain a chromophoric structure consisting of an extended conjugated system of p-electrons, often bearing groups that have an electron acceptor or donor [4], leading to enhanced hydrophilicity, anti-static, and UV protection [5,6]. Therefore, investigating the chemical and physical composition of natural dyes is necessary to introduce multiple active groups or chemical bonding on the surface of PET fabric [7,8]. Thus, it causes adjustments in the polymer chains of PET fabric, forming new stretching in their structure, leading to improved surface energy. Hybridization is an innovative way to prove the previously mentioned concept [9]. This research result provided a clear understanding of the interaction between Lawsone dye and PET fabric and achieved multifunction properties
Hybridization explores the relationship between the observed molecular shapes, the extent of surface area usable for intermolecular interactions [10,11], and the bonding characteristics of molecules [10,11]. Further, hybridization can contribute to the polarity of a molecule by affecting the electronegativity and arrangement of atoms [12,13]. These interactions are a form of intermolecular force and exhibit greater strength than London dispersion forces [14,15]. In addition, hybridization indirectly influences the occurrence of hydrogen bonding by influencing the arrangement of electronegative atoms, which play an essential role in hydrogen bonding. Since hybridization is affected by the type and properties of the solvent, selecting the proper solvent is a crucial factor that must be considered.
The solvent selection for extraction depends upon the specific chemicals being extracted, considering their solubility [16-18], polarity, and concentration [19]. Ethanol, a solvent possessing polar protic characteristics, exhibits selectivity in extracting compounds from different sources [20-22]. Water, containing a highly polar molecule because of the significant difference in electronegativity between oxygen and hydrogen, can dissolve polar and nonpolar compounds [23,24]. Due to their polarity, Both ethanol and water possess polarity, enabling them to dissolve polar molecules, create hydrogen bonds, and have higher boiling points than nonpolar compounds [25]. Moreover, their inherent polarity enables them to mix in different ratios, resulting in homogeneous mixtures.
Lawsone, derived from henna, a natural plant-based dye, is used in most textile materials [26,27]. Henna has astringent, moisturizing, and antimicrobial properties in traditional medicine and cosmetics [28,29]. Lawsone is a natural compound belonging to the naphthoquinone family with a structure that consists of a bonded ring system with a hydroxyl group (OH) attached to the aromatic rings, with double bonds of the carbonyl group (C=O) that contributes to its color and reactivity [30-32]. The double bond between carbon atoms and oxygen is responsible for molecule reactivity and polar covalent connections [33]. Nevertheless, using natural dyes to functionalize PET fabric has positively affected the environment and human health [34]. Although PET fabric is frequently hydrophilic, it has decreased surface energy, limiting the ability of moisture absorption [35,36]. Accordingly, the intermolecular forces between natural dyes during the dyeing process affect the PET fabrics, enhancing such properties to absorb water and increasing the dye uptake and other future properties.
The aim of the study is to produce LDE via different solvents in order to investigate the influence of hybrid LDE on the surface of PET fabric, with an objective of enhancing its hydrophilicity and anti-static properties. This has been achieved by a chemical composition. The surface morphology investigation was conducted to confirm the presence of LDE on the PET fabric. In addition, solvent extraction affects durability as measured via the K/S value. Their hydrophilicity and anti-static properties were characterized. Lastly, improvements associated with this technique make LDE a functional dye and environmentally friendly in the textile industry.
Experimental
Materials
PET Fabric was purchased from the Chinese market. Lwasone dye was brought from Sudan to China. Ethanol is provided by Aladdin Industrial Corporation, China. Deionized water was used without chemicals. We used all reagents without further purification.
Extraction Dye
The Lawsone dye powder was dried until it reached a stable weight. Then, an accurate measurement of 25 g was taken and dissolved in one litre of a different solvent for 24 hours. The initial solution preparation consisted of ethanol and water in a ratio of 10:90, and then deionized water was heated into vapour without using chemicals. After that, the solution was filtered to obtain the LDE solution. For comparative analysis, the color of LDE is different due to the oxidized state of the solvent used; boiled water displays a reddish-orange color, while mixed ethanol is brown. Figure 1A shows the chemical structure and predicted colors.
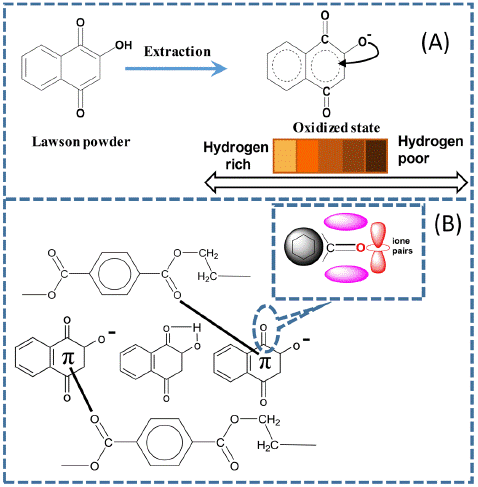
Figure 1: (A) Extraction behavior Lawsone (B) LDE on the PET fabric during the dyeing process.
Dyeing of Polyester Fabrics
The PET fabrics were dyed using an IR dyeing machine (Starlet 3). According to subsection 2.2, both extracts of the LDE solution had a liquor ratio of 30:1. The different dye temperatures were applied (100, 110, 120, and 1300 C) after cooling down. Subsequently, the colored PET fabrics were washed with deionized water. In addition, the sample was extracted with ethanol named PET-E fabric, while the other was PET-W fabric. In addition, washing fastness was performed in a washing test machine (SW-24A-I), following ISO 105-C10:2006 with a liquor ration of 40:1 and 5 g/L soap powder at 60°C for 30 min, and lastly washed with deionized water. After defining the best operational condition of the LDE process at the same concatenation, the dye bath was reused for cycle times using a standard sample and applying the previous methods.
Absorption Spectrum of Lawsone Dye Extracted
The LDE solution was prepared with different concentrations, and the absorption spectrum was analyzed using a Shimadzu UV-3600 ultraviolet-visible spectrophotometer. The wavelength range extended from 200 to 400 nm, and the optical data were obtained. The results were utilized to investigate the molecular and chemical composition.
Color Evaluation of K/S Value and Wash Fastness
The dyed fabric's color yield (K/S) values were determined using Datacolour 600 spectrophotometers (Datacolour Co., USA). They used artificial daylight (6500 K) at 10°, standard observers in the visible spectrum region 380–720 nm. Five different positions on the same dyed PET fabric were selected for testing, which measures the amount of light reflected and absorbed by a dye sample. Meanwhile, the washing fastness was carried out in the SW-24A tester for color fatness to washing (Wenzhou Darong Textile Instrument Co. Ltd., China), referring to AATCC 8-2008 (Colour Fastness to Crocking: AATCC Crockmeter Method).
Moisture Regain
We measured the moisture regain of dyed PET fabric. A PET fabric specimen was weighed and placed in an oven for 2 hours at 105°C. After 24 hours at room temperature, the conditioned dry fabric was considered. The moisture regain was obtained as follows:
Anti-static Property of Dyed PET Fabric
The YG (B) 342E-type fabric tester assesses the material's electrostatic characteristics. Sample sizes for PET-E and PET-W fabric were set up as follows: Electrostatic induction is observed when a PET fabric, sized 45 x 45mm, makes contact with another surface and gains an electric charge by transferring electrons [37]. Depending on the PET fabric characteristics, the charge can exhibit either a positive or negative polarity.
Characterizations of Chemical Compaction
The surface morphology of grafted PET fabric was observed using a Scanning Electron Microscope (SEM, TM-1000, Hitachi). The functional groups were characterized by Fourier Transform Infrared Spectroscopy (FT-IR); PerkinElmer Spectrum Two, the USA, in the range of 4000–400 cm1 at a resolution of 4 cm-1. X-ray diffraction (XRD, D2 Phaser, Bruker, Germany) investigated the crystal structures produced in the reflection mode. The Data color was created with (Data Color, Switzerland) under illuminant D65 using 10 standard observers in the visible spectrum region 380e 720 nm.
Result and Discussion
Mechanisms of Extraction and Dyeing Production
The experimental LDE was performed with different solvents to investigate the impact of LDE hybridization on the PET fabric surface. Ethanol is regarded as a solvent that selectively dissolves substances, whereas deionized water is utilized as a strongly polar solvent to extract both polar and nonpolar molecules [38]. In Figure 2, the UV-vis spectrum shows significant differences in dye absorption between ethanol and deionized water as solvents. During the dyeing with an infrared dyeing machine, the diffusion of dye molecules into PET fabric affects the chemical structures of LDE, such as double bonds conjugated carbonyl group (C=O) and a hydroxyl group (OH) attached to the aromatic rings, contribute through the hybrid orbital state in the formation of molecular orbitals and the arrangement of atoms within a molecule [39], including hydrogen bonding and dipole-dipole interactions (Figure 1), result: durable color in both PET-E and PET-W fabric with variations in color shade, this phenomenon because of solvent extraction. In addition, XPS defines the binding energies of the LDE carbonyl groups on the surface of PET fabric, showing noticeable changes due to intermolecular forces. These results agree with those obtained by decreasing WCA to zero and enhancing the anti-static properties, as shown in Figure 4.
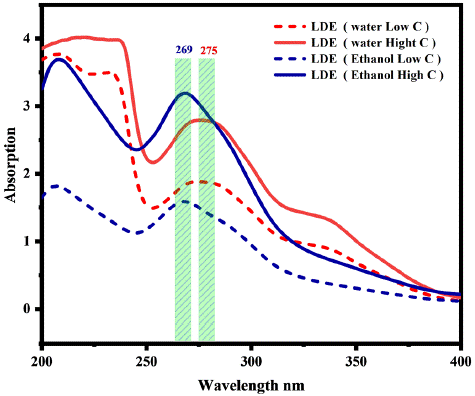
Figure 2: The absorption spectrum of LED liquid extract with different solvent.
Dissolution Behavior of Lwsone Dye Extracted
The extraction of LDE with different solvents (Section 2.2) considers polarity and solubility to investigate the absorption spectra. Consistently influence LDE via a hybrid state on the surface of PET fabric. Figure 2 displays the absorption peak at 269 nm, showing that ethanol exhibits higher absorption levels at high concentrations than deionized water. On the other hand, deionized water demonstrates greater extraction in low concentrations compared to ethanol, as demonstrated by the absorption peak observed at 275 nm. Consequently, the polarity idea proposes that deionized water higher polarity compared to ethanol allows it to extract even at low concentrations, explaining these findings. It is estimated that the difference in the chemical properties of these solvents can affect the PET fabric surface through the hybrid state and control the dye uptake, which produces a difference in color strength (Figure 5). In addition, the strong hydrogen bonding interaction and van der Waals forces between the dye molecules and PET fabric create a new hydrogen bonding interaction with the p-bond, thus improving the surface energy, as shown in Figure 1B.
Investigating the XRD and FTIR
X-ray Diffraction (XRD) is used to determine how the LDE is structured on the PET fabric and to compare the scattering intensities and angles of the PET fabric, the PET-W fabric, and the PET-E fabric, as shown in Figure 3A. The diffraction pattern exhibits different peaks observed at an angle of 215° to 30°. The PET fabric exhibits a medium peak, which matches the phase lattice parameter. After dyeing with LDE, the intensity peaks corresponding to PET-W fabric increased, while the intensity peak associated with PET-E fabric decreased. This change was due to the polarity and solubility of the solvent extraction [40]. XRD peak intensities might change due to surface energy variations brought on by the hybridization state. The oxidation state of LDE influences the PET fabric surface's lattice and geometry. That additionally has the potential to alter the crystal structure and packing of polymer chains, which can lead to various XRD diffraction patterns.
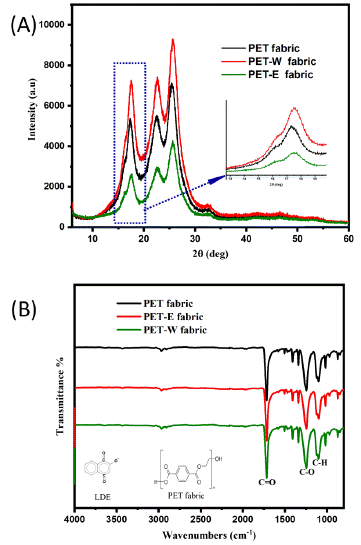
Figure 3: PET fabric before and after dyeing: (A) XRD, (B) FTIR.
In addition, The FTIR spectra of PET fabric before and after the dyeing process were tested and compared. Figure 3B shows the functional group spectra of PET, PET-W, and PET-E fabric. The absorption peaks of PET fabric at 1240 cm-1 and 1708 cm-1 correspond to the stretching vibrations of the C-O and C=O bonds, respectively. These vibrations are directly related to the stretching of ester groups. Hence, after dyeing PET fabric, the surface morphology remains unchanged. Perhaps the similarity in the molecular composition of both PET fabric and LDE is the reason for this. FTIR spectroscopy techniques are specifically developed to identify and analyze the functional groups present in chemical compounds. Therefore, the observed intensity peaks exhibited almost identical absorption vibrations. It can detect various absorption bands corresponding to particular functional groups present in a molecule.
Investigating the Effects of LDE Hybridization on PET Fabric
The structure of the carbon atom and the electronegativity of nearby atoms are two factors that affect the C1s binding energy, which stands for the core-level binding energy of the carbon atom [41]. Figure 4 shows fitting into three individual peaks in order to specify oxygen and hydrogen corresponding to the binding energy. In Figure 4A, PET fabric shows a strong peak at 407 eV related to C=O of the carbonyl group, while two peaks at 400 and 399 eV cross-ponding to C-O and C-H, respectively. After the dyeing process, Figure 4B illustrates that the PET-W fabric exhibits significant changes at 407 eV, attributed to the carbonyl group's presence. Additionally, there is a slight increase in the absorption peaks, contributing to C-O and C-H bonds. Furthermore, Figure 4C displays the branding peak of PET-E fabric attributed to the carbonyl groups with notable changes in C-O and C-H bonds. These changes indicated that the LDE successfully influenced the surface of PET fabric, which may have been caused by intermolecular force, increasing the surface energy. PET-E fabric showed a higher peak than PET-W fabric, which indicates that the solvent affects the surface.
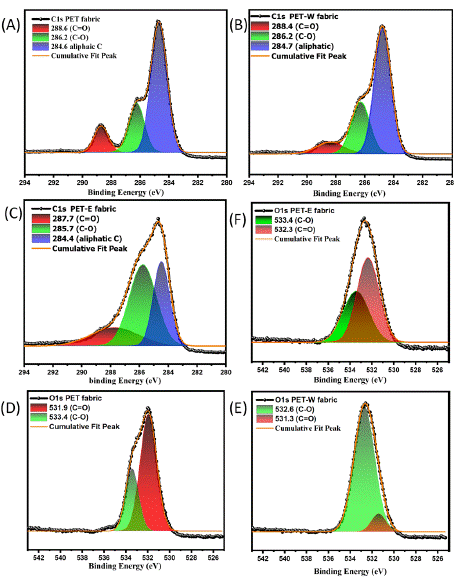
Figure 4: C1s XPS survey spectra of: (A) PET fabric, (B) PET-W fabric, (C) O1s XPS survey spectra of, (D) PET fabric, (E) PET-W fabric and (F) PET-E fabric.
To deepen our understanding of the influenced LDE hybrid, O1s have higher binding energy than C1s due to their more effective electron-binding properties, indicating their oxidation state alone does not determine O1s binding energy; additional factors like the chemical environment and electronegativity can also influence these binding energies. The O1's binding energy has been deconvoluted into two Gaussian components. In Figure 4D, PET fabric shows relative binding energy at 531.9 and 533.4 associated with C=O and C-O. After the dyeing process, in Figures 4E and 4F, the peak intensity shifted and changed in PET-W and PET-E fabrics, displaying that the carbonyl groups (C=O) decreased with increasing (C-O) bond. In contrast, Carbonyl groups (C=O) are polar due to the electronegativity difference between carbon and oxygen atoms, and this polarity gives rise to intermolecular forces, such as dipole-dipole interactions and hydrogen bonding, which can influence the surface and chemical properties of LDE. On the other hand, functional groups with C-O bonds also exhibit polarity due to the electronegativity difference between carbon and oxygen. It might be that increasing the peak related to C-O functional groups leads to a decrease in the carbonyl groups peak because of the delocalization of p electrons, as shown in Figure 1B. Lastly, as mentioned earlier, hybridization of LDE can affect the overall structure of the PET fabric; increasing the surface energy leads to a decrease in hydrophilicity and enhances the anti-static features.
Thermal Stability After Dyeing PET Fabric
A thermogravimetric investigation was performed to evaluate the thermal degradation and stability characteristics of PET, PET-W, and PET-E fabric [39]. During Thermal Gravimetric Analysis (TGA), the sample is exposed to high temperatures in a nitrogen environment [42]. Figure 5A shows that the stability remained unchanged before and after a dyeing procedure, as indicated by the absence of weight loss during TGA. Dyeing with LDE significantly indicates that it did not affect the thermal stability of the sample. According to the findings, this stability implies that the PET fabric does not undergo substantial chemical changes when exposed to the heating conditions associated with dyeing. The FTIR results indicated no new chemical bonds formed after dyeing with LDE. At the same time, the XPS fitting analysis supported the presence of intermolecular forces as the primary interaction between the LDE and the PET fabric. This suggests providing the successful penetration of the LDE molecules into the PET fabric surface through diffusion is primarily influenced by intermolecular interactions rather than the formation of covalent bonds.
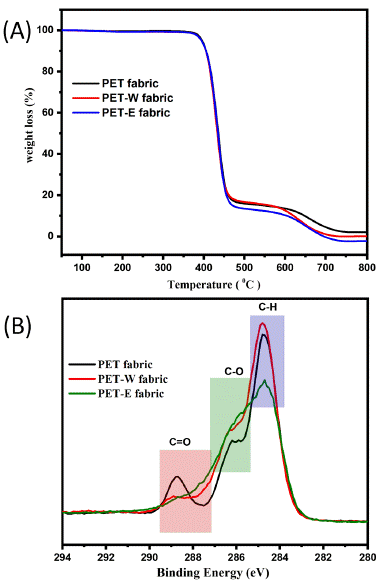
Figure 5: (A) TGA curves of and (B) XPS survey scan of O1s.
Colouring Durability
The color strength K/S value refers to the dye concentration required to produce a durable color with a considerable depth of shade [43]. Therefore, in Figure 6A, the data indicates that the K/S value of PET-E and PET-W fabric positively correlated with the dyeing temperature. This is due to the significant influence of temperature on dye absorption and color retention during the dyeing process [35]. Figure 6B displays the results of measuring the K/S coefficient with cyclic wash fastness. The PET-E fabric displays a higher K/S value, while the PET-W fabric demonstrates a slightly lower K/S value. However, both samples exhibited excellent durability against washing fastness. Indicate that the PET-E fabric has higher color strength and appears more vibrant than the PET-W fabric. The higher K/S values of PET-E fabric indicate that the colorants have better affinity and adherence; additionally, the modifications in the lattice and geometry of the PET fabric surface (section 3.3) impact the dyeing process due to the oxidation state of LDE. The surface energy and morphology changes can affect the interaction between the LDE molecules and the PET fabric, influencing the dye uptake and color intensity. Experimentally, the colorimetric indicators L*, a*, and b* are listed in Table 1, representing PET-E and PET-W fabric. A lower L* value corresponds to PET-E fabric, indicating a darker color. In comparison, PET-W fabric displays a higher L* value, indicating a brighter color. In addition, PET-W fabric displays positive values of a*, an indicator consistent with the principle of the effect of solvent extraction (reddish-orange color). Thus, the polarity of solvent extraction influences color shade through the hybridization state.
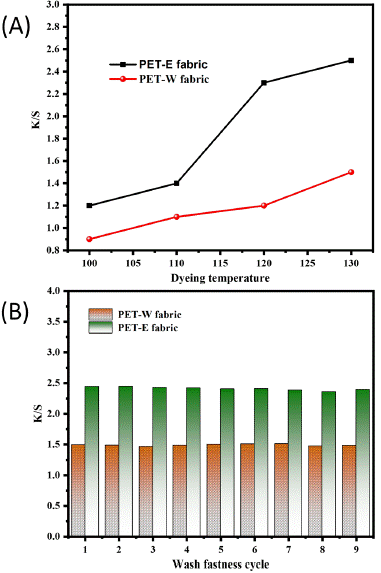
Figure 6: Evolution K/S value with: (A) dyeing temperature and (B) washing fastness.
Sample
K/S
L*
a*
b*
PET-E fabric
2.4436
8.41
-091
-2.50
PET-W fabric
1.5001
9.68
0.16
-1.16
Table 1: Colorimetric indicators for both solvent extracted.
Evalutin's Hydrophilicity Properties
PET is a synthetic polymer that is moisture-resistant. The hydrophobic nature of PET fabric is primarily attributed to its chemical structure, which lacks polar functional groups that readily interact with water molecules [44]. However, both surface roughness and intermolecular forces play a role in determining the wettability of the PET fabric surface. Figure 7(A) shows the WCA of PET fabric 120°. After dyeing with LDE on the surface, WCA decreased to become super hydrophilic, with a barely measurable and very small WCA. Diffusion of the LDE on the PET fabric improved the wettability due to increases in the surface roughness. In addition, Intermolecular forces, such as van der Waals forces and hydrogen bonding, can affect the interaction between a liquid and a PET surface. These forces can either enhance the wettability of the surface. In Figure 7(B), It was observed that a water droplet positioned on the surface of the PET fabric remained without spread and was absorbent for an extended period, compared to the PET fabric dyed with LDE.
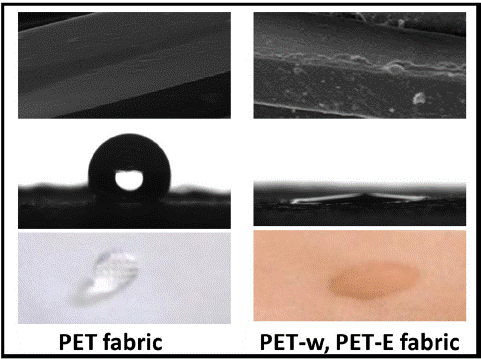
Figure 7: (A) water Contact angle with surface morphology (B) WCA evolution.
Evaluation of the Anti-Static Properties
The fabric's moisture absorption and retention characteristics are crucial for textile manufacturing and ensuring comfort in garments [45]. Hence, the moisture-related features of PET fabric are comparatively lower due to its hydrophobic nature, contributing to its performance and functionality [46]. In Figure 8(A), as reported in Section 2.6, the moisture regain of PET fabric was initially calculated as 0.6, which is attributed to its hydrophobic properties. However, after dyeing with LDE, there was a slight increase in moisture regain, which supports the previous decrease of WCA and can be attributed to the enhancement of surface energy. An investigation is being conducted to evaluate the electrostatic induction characteristics of PET fabrics in order to explore the impact of the hybridization of LDE [47]. Figure 8(B) illustrates the electrostatic induction characteristics related to changes in humidity. The PET fabric generates electrostatic charges within a limited range of 600 to 800. However, The PET-E and PET-W fabric both exhibited the generation of static electrostatic charge within long ranges of 123~1200 V and 100~1100 V, respectively.
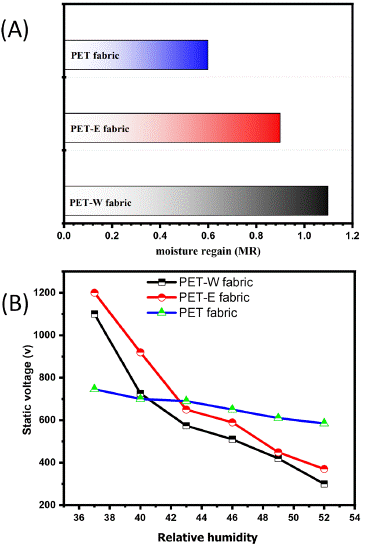
Figure 8: (A) moisture regain and (B) anti-static relative to moisture regain.
Conclusions
This work comprehensively investigates the effect of the Lawone molecule's hybridization on the surface of PET fabric. Hybridization through intermolecular force response increased the surface energy of PET-W and PET-E fabrics. Which increases the affinity to absorb moisture, confirmed by decreasing the WCA to zero degrees. Nevertheless, enhancing the moisture content leads to a decrease in static voltage. PET-E fabric has a higher static voltage (1200 V) than the PET-W fabric. Moreover, It was found that both extracted solvents influenced the dyed PET fabric, resulting in durable color with different color strengths. A higher K/S value related to PET-E fabric was observed. Meanwhile, PET-W fabric has higher and positive values of L* and a*, respectively, indicating a brighter color, suggesting that PET-W fabric can be considered a contrast color. Finally, this approach includes applying environmentally friendly materials, cost-effective techniques, and simple techniques that can be successfully utilized in comfort cloth production.
References
- Vankar, Gangwar, Archana. Natural dyeing mediated by atmospheric air pressure plasma treatment of polyester. J Pigment & Resin Technology. 2023.
- Xiang L, Chen H, Pang X, inventors; Hangzhou Huifeng Chem Fibre Co Ltd, assignee. Superfine anti-static polyester-cotton composite filament for fabric, comprises polyethylene glycol terephthalate, nylon, carbon nano-tube, carbon fiber, gutta-percha, crosslinking agent and coupling agent patent CN114703562-A; CN114703562-B.
- Sliman H, Dong X, Zhao T. Functionalization of polyethylene terephthalate knitted fabric with cowpea protein and biopolymer complex: Applications for enhancing wettability and UV-Protection properties. Journal of Colloid and Interface Science. 2020; 565: 360-7.
- Abdelghaffar F, Abdelghaffar RA, Rashed UM, Ahmed HM. Highly effective surface modification using plasma technologies toward green coloration of polyester fabrics. J Environmental Science and Pollution Research. 2020; 27: 28949-61.
- Erdogan MK, Akdemir Ö, Hamitbeyli A, Karakisla M. Preparation of hydrophilic woven fabrics: Surface modification of poly(ethylene terephthalate) by grafting of poly(vinyl alcohol) and poly(vinyl alcohol)-g-(N-vinyl-2-pyrrolidone). Journal of Applied Polymer Science. 2019; 137.
- Wang X, Lu D, Jonsson LJ, Hong F. Preparation of a PET-hydrolyzing lipase from Aspergillus oryzae by the addition of bis(2-hydroxyethyl) terephthalate to the culture medium and enzymatic modification of PET fabrics. Engineering in Life Sciences. 2008; 8: 268-76.
- DZea. Preparation and dyeing of super hydrophilic polyethylene terephthalate fabric. IOP Conf Ser: Mater Sci Eng. 2016; 137: 012051.
- Tang AYL, Lo CKY, Kan Cw. Textile dyes and human health: a systematic and citation network analysis review. Coloration Technology. 2018; 134: 245-57.
- Lewis DM, Broadbent PJ, Rigout MLA, Carr CM, Seaton CC, Swift T. Investigation into the development of novel lanthanide-based luminescent colorants for application to textiles and paper materials. Coloration Technology. 2023; 139: 610-20.
- Zezza P, Lucio MI, Fernandez E, Maquieira A, Banuls M-J. Surface Micro-Patterned Biofunctionalized Hydrogel for Direct Nucleic Acid Hybridization Detection. Biosensors-Basel. 2023; 13: 312.
- Yadav A, Varshney SK, Lahiri B. Hybridized Phonon Polaritons Assisted Broad Range SEIRA-Based Multimolecular Sensing. Ieee Sensors Journal. 2023; 23: 21812-20.
- Naval S, Verma P, Jain A, Mallick D. Hybrid vector and pressure sensor for fingertip dynamics sensing using DC-triboelectric/AC-piezoelectric mechanisms. Sensors and Actuators a-Physical. 2023; 355.
- Yu J. Multi-Scale Study on Strain-Hardening Cementitious Composites with Hybrid Fibers. 2021.
- do Nascimento KTO, Ratkovski GP, Pedro GdC, Gorza FDS, da Silva RJ, de Melo CP. Intrinsically conductive polymers hybrid bilayer films for the fluorescence molecular diagnosis of the Zika virus. Colloids and Surfaces B-Biointerfaces. 2021; 208: 112120.
- Mendonca Faria HA, Zucolotto V. Label-free electrochemical DNA biosensor for zika virus identification. Biosensors & Bioelectronics. 2019; 131: 149-55.
- Miftakhov RA, Lapa SA, Kuznetsova VE, Zolotov AM, Vasiliskov VA, Shershov VE, et al. Effect of Spacers on DNA Probe Properties in Hybridization Analysis. Russian Journal of Bioorganic Chemistry. 2021; 47: 1345-7.
- Avelino KYPS, Oliveira LS, Lucena-Silva N, Andrade CAS, Oliveira MDL. Flexible sensor based on conducting polymer and gold nanoparticles for electrochemical screening of HPV families in cervical specimens. Talanta. 2021; 226: 12118.
- Li C, Liang T, Lu W, Tang C, Hu X, Cao M, et al. Improving the anti-static ability of polypropylene fibers by inner anti-static agent filled with carbon nanotubes. Composites Science and Technology. 2004; 64: 2089-96.
- Zhao B, Han X, Hu C, Qian X, Duo Y, Wang Z, et al. Hydrophilic Modification of Polyester/Polyamide 6 Hollow Segmented Pie Microfiber Nonwovens by UV/TiO2/H2O2. Molecules. 2023; 28: 3826.
- Adedokun O, Sanusi YK, Awodugba AO. Solvent dependent natural dye extraction and its sensitization effect for dye sensitized solar cells. Optik. 2018; 174: 497-507.
- Sabarikirishwaran P, Unpaprom Y, Ramaraj R. Effects of Natural Dye Solvent Extraction on the Efficiency of Dye-Sensitive Solar Cells from the Leaf Biomass of Sandoricum koetjape and Syzygium samarangense. Waste and Biomass Valorization. 2023; 14.
- Loum J, Byamukama R, Wanyama PAG. Efficient Extraction of Natural Dyes from Selected Plant Species. Chemistry Africa. 2021; 4: 677-89.
- Mei Y, Deskins NA. An evaluation of solvent effects and ethanol oxidation. Physical Chemistry Chemical Physics. 2021; 23: 16180-92.
- Ravagnani MASS, Reis MHM, Filho RM, Wolf-Maciel MR. Anhydrous ethanol production by extractive distillation: A solvent case study. Process Safety and Environmental Protection. 2010; 88: 67-73.
- Sivakumar V, Vijaeeswarri J, Anna JL. Effective natural dye extraction from different plant materials using ultrasound. Industrial Crops and Products. 2011; 33: 116-22.
- Atav R, Namirti O. An Ecofriendly Dyeing Method for Polyester Fibers: To Bring Traditional Natural Dyeing into Industrial Production. Fibers and Polymers. 2023; 24: 2027-38.
- Fang J, Meng C, Zhang G. Agricultural waste of Ipomoea batatas leaves as a source of natural dye for green coloration and bio-functional finishing for textile fabrics. Industrial Crops and Products. 2022; 177: 114440.
- Bhuiyan MAR, Islam A, Ali A, Islam MN. Color and chemical constitution of natural dye henna (Lawsonia inermis L) and its application in the coloration of textiles. Journal of Cleaner Production. 2017; 167: 14-22.
- Rubio L, Costa M, Barrulas P, Lores M, Garcia-Jares C, Barrocas-Dias C. Understanding the chemical and mineralogical composition of commercial henna and jagua tattoos and dyes—a multi-analytical approach. Analytical and Bioanalytical Chemistry. 2022; 414: 6233-46.
- Badoni Semwal R, Semwal DK, Combrinck S, Cartwright-Jones C, Viljoen A. Lawsonia inermis L. (henna): Ethnobotanical, phytochemical and pharmacological aspects. Journal of Ethnopharmacology. 2014; 155: 80-103.
- Ebrahimi I, Gashti MP. Extraction of polyphenolic dyes from henna, pomegranate rind, and Pterocarya fraxinifolia for nylon 6 dyeing. Coloration Technology. 2016; 132: 162-76.
- Blackburn RS, Bechtold T, John P. The development of indigo reduction methods and pre-reduced indigo products. Coloration Technology. 2009; 125: 193-207.
- Alam MM, Rahman ML, Haque MZ. Extraction of Henna Leaf Dye and its Dyeing Effects on Textile Fibre. 2007; 42.
- Zhang B, Chen S, Wang W, Tian M, Ning N, Zhang L. Polyester (PET) fabrics coated with environmentally friendly adhesive and its interface structure and adhesive properties with rubber. Composites Science and Technology. 2020; 195: 108171.
- Nahar N, Heng Q, Sadi MS. Surface Modification of Non-Ionic Polyester Fabric into an Anionic Platform for Low Temperature Cationic Basic Dyeing with Improved Colorfastness Properties. Fibers and Polymers. 2023; 24: 1345-57.
- Ghamarpoor R, Jamshidi M, Sayyadian M, Razavizadeh M. Chemical/photochemical functionalization of polyethylene terephthalate fabric: effects on mechanical properties and bonding to nitrile rubber. Scientific Reports. 2023; 13.
- Zhang X. 2 - Anti-static and conductive textiles. In: Pan N, Sun G, editors. Functional Textiles for Improved Performance, Protection and Health: Woodhead Publishing. 2011; 27-44.
- Louzi VC, Campos JSdC. Corona treatment applied to synthetic polymeric monofilaments (PP, PET, and PA-6). Surfaces and Interfaces. 2019; 14: 98-107.
- Jiang Z, Li H, Zhang C, Zhu P. N-substituted cyclic phosphoramides as flame-retardant and anti-dripping coatings for PET fabric. Surfaces and Interfaces. 2022; 34: 102409.
- Liu Z, Li L, Zhao Z, Liu Y, Lu M. Anti-static silk fabric through sericin swelling-fixing treatment with aminated carbon nanotubes. Materials Science and Engineering: B. 2017; 226: 72-7.
- Bayoumi EE, Attia NF, Elshehy EA, Abd El-Magied MO, Atia BM, Galhoum AA, et al. Tungsten-based hybrid nanocomposite thin film coated fabric for gamma, neutron, and X-ray attenuation. Surfaces and Interfaces. 2023; 39: 102883.
- O’Flynn K, Milosavljevic V, Dobbyn P, Dowling DP. Evaluation of a reel-to-reel atmospheric plasma system for the treatment of polymers. Surfaces and Interfaces. 2017; 6: 162-9.
- Burkinshaw SM. The roles of elevated temperature and carriers in the dyeing of polyester fibres using disperse dyes: part 3 model of dye adsorption based on dye solubility. Coloration Technology. 2023.
- Raman A, Jayan JS, Deeraj BDS, Saritha A, Joseph K. Electrospun Nanofibers as Effective Superhydrophobic Surfaces: A Brief review. Surfaces and Interfaces. 2021; 24: 101140.
- Su M, Chen X, Zhang L, Min J. Synthesis of Active Graphene with Para-Ester on Cotton Fabrics for Anti-static Properties. Nanomaterials. 2020; 10.
- Liu Z, inventor; Liu Z, assignee. Producing anti-static polyester fabric involves dispersing nano-silica in solvent, and adding high molecular weight polylactic acid for dehydration and polycondensation to obtain polymer dispersion solution patent CN112267155-A.
- Wang Z, Wang D, Zhu Z, Li W, Xie Y. Enhanced anti-static properties of polyethylene film/polypropylene-coated non-woven fabrics by compound of hot-melt adhesive and polymer anti-static agent. Journal of Industrial Textiles. 2019; 50: 921-38.