
Research Article
Adv Res Text Eng. 2023; 8(1): 1082.
Modification of Jute Fiber with Vinyl Acetate and Methyl Vinyl Ketone for Textile Applications
Md. Hasinur Rahman; Md. Jahangir Alam; Md. Ibrahim H Mondal*
Polymer and Textile Research Lab, Department of Applied Chemistry and Chemical Engineering, Rajshahi University, Rajshahi-6205, Bangladesh
*Corresponding author: Md. Ibrahim H Mondal Polymer and Textile Research Lab, Department of Applied Chemistry and Chemical Engineering, Rajshahi University, Rajshahi-6205, Bangladesh. Email: mihmondal@gmail.com; mihmondal@yahoo.com
Received: June 08, 2023 Accepted: July 07, 2023 Published: July 14, 2023
Abstract
In the present study, the bleached jute fiber was been modified with Vinyl Acetate (VA) and Methyl Vinyl Ketone (MVK) by graft co-polymerization in an aqueous medium using potassium persulphate as an initiator, under the catalytic influence of FeSO4 in the presence air to improve physicochemical properties. The maximum graft yield and grafting efficiency at the optimized condition for VA and MVK are 26.0%, 11.1% and 10.4%, 3.7% respectively. The graft yield and grafting efficiency of VA-modified fiber were higher than that of MVK-modified field fiber. The grafted fiber was characterized by FTIR, SEM and TGA. FTIR spectra of VA and MVK modified fiber have peaks at 1741cm-1 and 1714cm-1 respectively than the unmodified jute indicating the incorporation of vinyl monomer with the cellulosic jute fiber. By observing thermograms, the degradation temperature of bleached jute fiber was 318.7°C and that for VA and MVK modified fibers was 320°C and 325°C respectively. The breaking strength of bleached fiber was 16.02kg/yarn but VA and MVK modified fibers were 17.3 and 17.00kg/yarn respectively. The dye absorption by modified fiber was slightly decreased than bleached fiber due to the increased hydrophobicity. The wash fastness with soap solution decreased with the increase in washing temperature. The color fastness test of dyed bleached and modified jute fibers spotting with alkalis, and acids were satisfactory with some exceptions. Thus, the modification improves the physicochemical properties of the jute fiber for textile applications.
Keywords: Jute fiber; Vinyl acetate; Methyl vinyl ketone; Grafting; Textile application
Introduction
Textile is a primary requirement of human beings. Humans started using clothing to protect their bodies from adverse conditions, to look civilized and distinguished and to feel comfortable. With progress in time, society began to demand multifunctionality in clothing, and thus, in today’s innovative world, value addition is very much sought after [1]. Fabrics were therefore treated chemically or mechanically to get various functionality on them [2]. The functional modifications and speciality properties of textiles rely closely on their surface chemical and physical structures that vary in keeping with variations in the polymer composition, structures of fibers, and fiber assemblies and their treatment [3-5]. Textile fibers are natural or synthetic structures that can be spun into yarn and woven, knitted, or bonded into the fabric [6]. As a consequence of growing concerns related to the adverse effects of synthetic materials on the environment and human ecology, natural fibers attract more and more interest as an alternative to petro-based counterparts [7,8]. Aside from being renewable, biodegradable, and nontoxic, natural fibers have a variety of other advantages, including availability, low density, non-abrasiveness, combustibility, increased energy recovery, higher CO2 sequestration, and cost efficiency [3,9,10].
Jute (Corchorus sp.) is a major source of natural fibers, which represent about 80% of the global production of bast fibers [11]. It is a natural, long, and soft vegetable fiber with a golden silky shine also termed Golden Fiber. Lignin, cellulose, waxes, pectin, protein, nitrogenous chemicals, and mineral and inorganic substances make up the majority of the components of jute fiber [12-14]. Jute fibers are completely biodegradable and recyclable materials, i.e. environmentally friendly [15]. It is a lignocellulosic, multicellular bast fiber which is mostly utilized for packaging as well as other varied textile and non-textile applications, including technical textiles. Decorative upholstery items and household textiles made of jute are now also utilized for furniture [12,16]. The most important fashionable feature of jute products is the natural golden color that provides an elegant look and aesthetics [17]. In addition to its technical benefits including high tensile strength, initial modulus, moisture recovery, outstanding sound and heat insulation characteristics, good dimensional stability, harmlessness and good dye acceptance, jute fiber is agro-renewable, biodegradable, and easily accessible at affordable prices [18,19]. It also has certain disadvantages including relative coarseness, brittleness and harshness in feel, considerable range in fiber length and fineness, and susceptibility to yellowing when exposed to sunshine [19,20]. Researchers have been working to address the inherent shortcomings of jute fiber by employing graft co-polymerization with a variety of monomers, including acrylate [14,21,22], amide [13,23], silane [24], acrylic acid [25], nitrile [26], etc. However, most of the time, it was demonstrated that each monomer only contributed to solve one specific problem. Therefore, researchers are looking for a particular modifying agent that can help to reduce all the drawbacks of jute fiber in order to make it more sustainable. In the present work, the effect of grafting Vinyl Acetate and Methyl Vinyl Ketone onto jute fiber has been investigated. The textile characteristics of the grafted fibers, e.g. dyeing properties, tensile properties, thermal stability, and fastness properties of jute fibers are also studied.
Experimental
Materials: Jute fiber [Corchorus Olitorious (Tossa variety)] was collected from the local market in Bangladesh. Anhydrous sodium carbonate, sodium chlorite (NaClO2, 80%), sulphuric acid (H2SO4,98%), formic acid (90%), ferrous sulphate (FeSO4, 7H2O, 99.5%), vinyl monomers [Vinyl Acetate (VA) and Methyl Vinyl Ketone (MVK)] were purchased from BDH (England). Dyestuffs were purchased from Sigma-Aldrich (USA). Glacial acetic acid (CH3COOH, 99.7%), sodium meta-bisulphite or sodium bisulphite (Na2S2O5, 99.9%) was purchased from Merck, German.
Method of Modification of Jute Fiber
The graft co-polymerization of bleached jute fiber was carried out in a 100ml stoppered Erlenmeyer flask. Polymerization was done with 250-300 % monomer, 21-22% potassium persulfate as initiator, and 5-8% ferrous sulfate as the catalyst based on the weight of the fiber, at 80-90 °C for 120 min, in the fiber-liquor ratio of 1:30. At the end of the desired reaction, the fibers were washed with hot distilled water to remove the attached homo-polymer on the jute sample and dried at room temperature [13,14].
Determination of Percent Graft Yield and Grafting Efficiency
Graft yield percentage and grafting efficiency percentage were calculated according to the following formula [21].
Graft yield (%) =
Grafting efficiency (%)
Where A is the weight of the grafted jute fiber after modification, B is the weight of ungrafted jute fiber before modification and C is the weight of the total monomer used.
Moisture Content Study
One gm of jute sample was dried at 105°C in an electric oven for one hour and then the sample was kept in a desiccator for 30 min and finally, the sample was weighed. The difference in weight gave the amount of moisture. The percentage of moisture is calculated by the following formula [13,14]:
Moisture, % =
Where A is the initial weight of the sample and B is the weight of the moisture-free sample.
Fourier Transform Infrared (FTIR) Analysis
FTIR spectra of bleached and modified jute fibers were determined using Shimadzu-8900, FTIR Spectrophotometer (Kyoto, Japan). Samples were placed in the path of an infrared beam of wave number in the range of 400-4000 cm-1.
Thermal Behavior
Comparative thermal stabilities of bleached and modified jute fibers were studied using a Perkin Elmer simultaneous thermal analyzer (STA 8000, Germany). The tests were conducted between 30-500oC under an inert atmosphere (Nitrogen). The heating rate and the airflow rate were 20° C/min and 200 ml/min respectively.
Scanning Electron Microscopy (SEM)
Scanning Electron Microscopy (SEM) was used to observe the microstructure and the surface morphology of treated as well as untreated jute fiber. SEM micrographs were obtained by FEI Quanta Inspect, Model: S50 and samples were coated with silver.
Colour Fastness Test: Sunlight, Water, Acid and Alkali
Color fastness to acids, alkalis, sunlight and washing with soap solution of bleached, VA and MVK modified dyed jute fibers was assessed by Greyscale.
Colour Fastness to Sunlight
A light fastness test was carried out on both the dyed and undyed bleached and modified jute fibers. Specimens of the fibers were attached separately on a board by a glass rod and placed under the sun in open air for six hours each day and continued for 200 hours without any protection from weathering, but were protected from rain, dew, etc. After every 40 hours, the change in color of the specimens was assessed by the Greyscale with respect to control [14,21].
Colour Fastness to Washing
A bath was prepared with 5gm of wheel soap per liter of distilled water. The fiber liquor ratio was 1:50. One gram of dyed jute fiber of length 10 cm was entered in the bath and the bath temperature was maintained at 40°C for 30 minutes. The bath solution was kept under agitation. After treatment the fiber was washed thoroughly with distilled water and dried in air at room temperature. The change in color was assessed with the Greyscale. Similarly wash fastness was assessed at 60°, 80°, 90° and 100°C [9, 27].
Colourfastness to Acid and Alkali Spotting
1. Acid, alkali, undyed and dyed bleached and modified jute fibers were combed and compressed enough to form a sheet 10cm×4cm. The specimens were spotted with two drops of acid or alkali solution at room temperature. The surfaces of the specimens were gently rubbed with the glass rod to ensure penetration. The specimens were dried hanging them in air at room temperature. The change in color of the specimens was assessed after drying with Grey scale [9,27]. In the same way, the change in color was assessed by the solution given belHydrochloric acid solution containing 50 gm per liter.
2. Acetic acid solution containing 300 gm of glacial acetic acid per liter.
3. Sodium carbonate solution containing 100 gm anhydrous sodium carbonate per liter.
4. Sodium hydroxide solution containing 50 gm of sodium hydroxide per liter.
5. Ammonia solution containing 10% of ammonia.
6. Formic acid solution containing 300 gm per liter.
Results and Discussion
Dyeing Behavior and Mechanism of Modification
Bleached jute fiber was chemically modified using vinyl acetate and methyl vinyl ketone monomers at varied monomer, catalyst, and initiator concentrations, duration, and temperature. The percent graft yield increased with an increase of monomer concentration up to 250% for VA and 300% for MVK and thereafter decreased (Figure 1). It is evident that the increase in monomer concentration in an aqueous medium increased the reaction sites and complexation of jute with VA and MVK enhanced reactivity due to the formation of donor-acceptor complex. However, increased monomer concentrations in the polymerization medium favored molecular collision, thereby enhancing polymerization in general or homopolymer formation in particular. As a result, the decrease in graft yield at a given monomer concentration suggests that homo-polymerization prevails over grafting at higher VA and MVK concentrations [21,22,28].

Figure 1: Effect of monomer concentration grafting of VA and MVK onto jute fiber.
Figure 2 demonstrates that the graft yield and grafting efficiency of bleached jute fiber increased with temperatures up to 90°C for VA and 80°C for MVK, but once these temperatures were achieved, they rapidly decreased. The increased graft yield may be ascribed to an increase the rate of production of active free radicals, which increases the number of grafting sites at a higher rate and increase the rate of diffusion of monomers (VA and MVK) into the fiber matrix [22]. The decrease in graft yield beyond 90°C for VA and 80°C for MVK may be attributed to the increase in activation energy for graft co-polymerization and premature termination of growing grafted chains by excess Fe(III) ions produced on oxidation of Fe(II) ions [21,29].

Figure 2: Effect of modification temperature on grafting of VA and MVK onto jute fiber.
The graft yield and grafting efficiency improved with reaction time up to 90 minutes for VA and 80 minutes for MVK, then declined to some extent, as indicated in Figure 3. The rate of grafting increased with time at first very sharply due to maximum co-polymerization reaction and a minimum homo-polymerization reaction. The percent graft yield decreased with increasing reaction time due to the partial breakdown of grafted fiber [21,30].
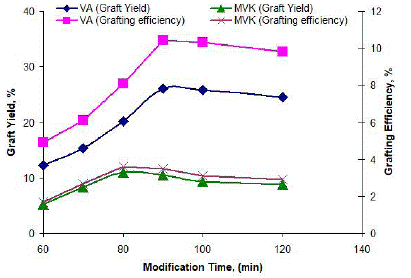
Figure 3: Effect of modification time on grafting of VA and MVK onto jute fiber.
Increasing the potassium peroxodisulphate concentration as an initiator boosted graft yield and grafting efficiency up to 21% for VA and 22% for MVK due to the creation of a large number of active sites on the backbone of the jute fiber, as shown in Figure 4. The retarding effects of the graft yield and grafting efficiency at higher initiator (K2S2O8) concentration may be ascribed to, the predominancy of homo-polymerization over grafting, The termination of growing grafted chain by primary free-radicals resulting from the decomposition of the excess of the initiator, and the production of excess Fe(II) ions which interact with the growing grafted chains leading to the cease of their growth [14,31].

Figure 4: Effect of initiator concentration grafting of VA and MVK onto jute fiber.
Under optimized conditions, the maximum graft yields were 26% and 11.1% and grafting efficiency was 4% and 3.7% respectively for VA and MVK-modified jute fibers (Figures 1-4). The graft yield and grafting efficiency of VA are higher than those of MVK. This is due to the different chemical structures of Vinyl Acetate (VA) and Vinyl Methyl Ketone (MVK). In this two-vinyl monomer, there are two same vinyl group. But in Vinyl Acetate (CH2=CHCOOCH3) there is one extra oxygen atom with Crabonyl (CO) compared to Methyl Vinyl Ketone (MVK). Due to this extra oxygen in Vinyl Acetate, carboxyl carbon (CO) become more electrophilic than MVK’s carbonyl carbon. For this reason, vinyl acetate reacts with jute radicals more easily than the MVK [32,33].
The proposed mechanism of graft co-polymerization of vinyl monomers (VA and MVK) onto bleached jute fiber initiated by potassium persulphate, K2S2O8 in the presence of FeSO4 may be pictured as involving the generation of macro-radical (-OH and SO4) species and to some extent by Fe(III) ions on jute fibers. The free radical species and Fe(III) ions are produced from the redox reaction between K2S2O8 and Fe(II). The macroradicals so formed subsequently attack the monomer leading to chain initiation. The termination of graft co-polymerization may be due to the interaction with Fe(III) ions or mutual combination of grafted chains on different backbones [14,23,34].
FTIR Analyses
The FTIR spectra of bleached jute fiber and grafted fibers are represented in Figure 5. The absorption peak appears at 1741 cm-1 for VA as a result of the ester group and at 1714 cm-1 for MVK as a result of Ketone group stretching in the grafted fibers [32,33]. This indicated that the incorporation of vinyl monomers with jute fiber occurred.
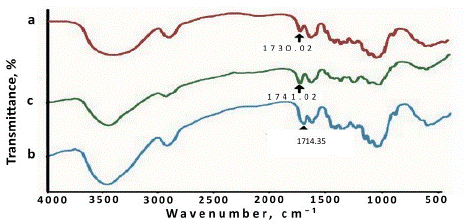
Figure 5: FTIR spectra of a) Bleached jute fiber: b) MVK-modified fiber and c) VA-modified jute fiber.
Surface Morphology
Figure 6 shows the surface morphologies of bleached and modified jute fibers respectively. The surfaces of the modified fibers were homogeneous, smooth, and continuous, no pores appear whereas the surface of unmodified bleached fiber was irregular, ruptured and cracked [14,22]. The modification of tightly bound irregular and rough surfaces of jute is observed through grafting or with interpenetrating cross-linking, and finally formed a smooth surface morphology [2,21,27].

Figure 6: SEM of (a) bleached, (b) VA modified, and (c) MVK modified jute fibers.
Thermal Analysis
Figure 7 shows TGA thermograms of bleached, VA and MVK modified jute fiber. According to TGA thermograms, the thermal stability of jute fibers shows the following orders: bleached < VA modified < MVK modified jute fiber. It is observed that the loss in weight up to 110°C for bleached fiber is around 5.0% and that for VA and MVK-modified fibers are 3.2% and 3.2%, respectively. The actual pyrolysis region of bleached fiber is 318.7°C to 373.5°C and that for the VA and MVK modified fibers are 320°C to 379°C and 325°C to 384.1°C respectively. The corresponding weight loss was 66.2%, 79.1% and 76.0%, respectively. Thus the thermal stability of VA and MVK modified fibres were slightly increased than unmodified fibre which might be happened due to the incorporation of vinyl monomers with the fibre. The weight loss was due to the removal of volatile substances and moisture from the fiber and the pyrolysis caused the partial degradation of the cellulosic backbone and the complete removal of hydrogen from the structure [32,33].
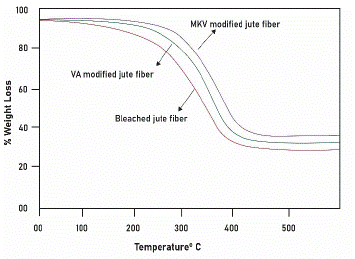
Figure 7: TGA thermograph of bleached, VA and MVK-modified jute fibers.
Between unmodified and modified fibers, the latter showed better thermal stability. This can be attributed to the incorporation of vinyl monomers (VA and MVK) into the fiber surface that forms a protective layer on the active surface of the jute fiber. Initially, this layer counteracted the applied heat, ultimately reducing the heating effect on the fiber backbone [32). Therefore, the modified fiber exhibited higher thermal stability. Between VA and MVK-modified fibers, the latter showed significantly improved results. The better thermal performance of the MVK-modified fibers was due to the formation of intra-/inter-cellulose units crosslinked by MVK.
Moisture Content
The bleached jute fiber had a moisture content of 7.37%. The moisture content of VA and MVK-modified fibers (6.9% and 6.0%, respectively) were slightly decreased due to increasing the fiber hydrophobicity. The moisture contains MVK grafted fibers are comparatively higher than that of VA grafted fibers due to the high graft yield (26%) of vinyl acetate than that of methyl vinyl ketone (11.1%) to jute fiber [13,14].
Mechanical Strength
The breaking strength of bleached, VA and MVK-grafted fibers were 16.02, 17.3 and 17.0kg/yarn respectively. It is seen that the breaking strength of modified jute fiber was higher than that of the bleached fiber. This is caused due to grafting of jute fiber with VA and MVK which may reinforce the fiber and consequently strengthen the grafted jute fiber [14,35].
Dyeing Characteristics
Table 1 shows the dyeing and the dye absorption value of bleached and modified jute fibers. The bleached fiber absorbed a higher amount of dye than grafted fiber. The possible explanation for this difference in dye exhaustion is that the Bleached jute fibers had more available pores or voids than modified jute fibers that were blocked by hydrophobic vinyl monomers during grafting. Thus, the availability of pores or cavities for dye penetration was reduced and dye absorption by grafted fiber was lower than that of bleached jute fiber [16,21,31,36].
Dyes
Name of dye
Dye exhaustion, %
Beached fiber
VA-grafted fiber
MVK-grafted fiber
Reactive dyes
Reactive Orange 14
46.3
42
39.8
Reactive Brown 10
45.0
43.2
39.0
Direct dyes
Direct Orange 31
89.5
86.7
82.5
Direct Blue 1
95
93.22
89.3
Table 1: Effect of dye absorption on dyeing of bleached and modified (VA and MVK) jute fiber.
Colour Fastness of Modified Jute Fiber
The color fastness of both the bleached and modified (VA and MVK) jute fibers, dyed with Direct Blue 1, Direct Orange 31, Reactive Brown 10, and Reactive Orange 14 were studied on exposure to sunlight in the open air and on washing with soap solution respectively by the Greyscale. The color fastness of undyed and dyed bleached jute fibers to spotting with organics acids, such as formic acid, and acetic acid is excellent in all cases. But in the case of modified jute with VA and MVK, when hydrochloric acid was used for the spotting test, a slight change in color occurred. This is due to the fact that jute is not resistant to strong acids and produces hydrocellulose on acid hydrolysis. Cleavage of cellulose chains by hydrolysis breakdown the covalent bonds between the fabric and dyes, therefore in the presence of hydrochloric acid the color fastness of dyed modified jute fibers are not at all satisfactory [9,31]. Color fastness of unmodified and modified jute fiber is excellent in most circumstances for weak alkalis such as NH4OH, but with NaOH and Na2CO3, it offers unsatisfactory results by altering color. This is because the lignin and hemicellulose found in jute fiber are extremely reactive with strong alkalis, and their reactivity depends on the strength of the alkali [9,14,31].
The wash fastness of colored jute fiber at 40, 60, 80, and 100 °C was investigated using direct and reactive dyes. The dyed fibers fade when washed with soap solution, however, they were found to be good at lower temperatures, but the wash fastness declined as the washing temperature increased. It appears that at higher temperatures, the dissolution of the dye particles from the fiber surface takes place and hence more dye particles are easily washed off the fabric [31]. It has also been observed that among the direct dyes, Direct Blue 1 retains its color on bleached and modified jute fiber to a large extent after washing with a soap solution. This is because the wash fastness of a fiber is determined by its physical and chemical properties, the type of dye and the forces that interact with it, as well as its interaction with a soap solution. T Direct Blue-1's superior capacity to establish covalent bonds with fiber molecules may account for improved color fastness when washed with a soap solution [37]. Furthermore, Reactive Orange 14 has slightly better color fastness when washed with soap solution than Reactive Brown 10 due to a structural feature of dyes in which the number and strength of covalent bonds between Reactive Orange 14 and the fiber molecules are greater than those of Reactive Brown 10 [37].
The lightfastness rating of bleached and modified jute fibers after 200 hours of exposure to sunlight in open air is nearly the same. However, the colourfastness of modified fiber was somewhat better than that of bleached fiber. This could be because modification formed a protective layer on the jute surface. This protective layer may have partially protected the jute from UV rays penetrating the fiber backbone. This effect reduces the negative effects of UV light on color fastness. Again, this protective layer of modification reduces the diffusion of moisture and oxygen both of which are known to participate in fading or yellowing [14,20]. It is observed that jute fibers dyed with Reactive Orange 14 in the presence of electrolyte [Al2(SO4)3] exhibit considerably better color fastness on exposure to sunlight in the air than the fiber dyed with Reactive Brown 10. This may be due to the structural features of the dyes. The dichlorotriazinyl group of reactive dyes has two electron-deficient carbon groups and is therefore, susceptible to nucleophilic substitution reaction with partially ionized hydroxyl ions of the cellulose can be formed by either nucleophilic substitution or addition and the presence of more in number of these bonds will give better color fastness properties. From the structure of dyes, it is observed that Reactive Orange 14 contains one dichlorotriazinyl group where as, Reactive Brown 10 contains one monoclorotriazinyl group. Thus, the number of covalent bonds between Reactive Orange 14 and the jute cellulose will be more and stronger than that of Reactive Brown 10. So, Reactive Orange 14 exhibits better color fastness on exposure to sunlight [37].
Similarly, jute fiber dyed with Direct Blue 1 has significantly superior color fastness in the air than Direct Orange-31. Color fastness of Direct Blue 1 is slightly improved it has excellent covalent bond-forming capacity. The fastness of a dyed fabric is determined by the interaction of the dye with the fiber and the intensity of the light. The capacity of the dye molecule, stimulated by the light, causes intense oxidation of the fiber resulting in color change. The change in color of the dyed jute fiber is obvious due to the mechanism of light action induced by the dye on the fiber [31].
Conclusion
The graft yield and grafting efficiency of vinyl acetate and vinyl methyl ketone into jute increases with the increase of monomer concentration, initiator concentration, catalyst concentration, modification time and temperature up to a certain value and then decreased. The thermal stability of the modified fiber is improved. The moisture absorption capacity of modified fiber decreases i.e. hydrophobic nature of modified jute fiber increased. The breaking strength of modified jute fiber is higher than that of bleached jute fiber. In the case of dyeing, the percent of dye absorption of modified fiber slightly decreased than that of bleached fiber. The color fastness of modified jute fibers is slightly better than that of bleached jute fiber. This research indicates that modifying jute with VA and MVK has a positive impact on textile performance.ow:
References
- Kalia S, Thakur K, Celli A, Kiechel MA, Schauer CL. Surface modification of plant fibers using environment friendly methods for their application in polymer composites, textile industry and antimicrobial activities: a review. J Environ Chem Eng. 2013; 1: 97-112.
- Rachini A, Le Troedec ML, Peyratout C, Smith A. Chemical modification of hemp fibers by silane coupling agents. J Appl Polym Sci. 2012; 123: 601-10.
- Thakur VK, Thakur MK. Processing and characterization of natural cellulose fibers/thermoset polymer composites. Carbohydr Polym. 2014; 109: 102-17.
- Khedkar SS, Mallick ASC. Aroma textilis. In: Shahid ul-Islam and Singh B (Bds.), Advances in Functional and Protective Textiles. 2020; 169-90.
- Thakur VK. Thakur M Kessler MR. In: Thakur VK, Thakur MK, Kessler MR, editors. Handbook of composites from renewable materials. Scrivener publishing. Okra bast fiber as potential reinforcement element of biocomposites: Can it be the flax of the future. 2017; 4: 379-405.
- Khan GMA, Saheruzzaman M, Razzaque SMA, Islam MS, Alam MS. Grafting of acrylonitrile monomer onto bleached okra bast fibre and its textile properties. Indian J Fibre Text Res. 2009; 34: 321-7.
- John MJ, Anandjiwala RD. Recent developments in chemical modification and characterization of natural fiber-reinforced composites. Polym Compos. 2008; 29: 187-207.
- Bismarck A, Mishra S, Lampke T. Plant fibers as reinforcement for green composites. In: Mohanty AK, Misra M, Drzal LT, editors. Natural fibers, biopolymers, and biocomposites. Boca Raton: Taylor & Francis; 2005; 37-108.
- Khan GM. Dyeing of grafted jute fibre with reactive dyes and its improved properties. Thammasat Int J Sci Technol. 2007; 12: 83-7.
- Lee TS, Choi HY, Choi HN, Lee K-Y, Kim S-H, Lee SG et al. Effect of surface treatment of ramie fiber on the interfacial adhesion of ramie/acetylated epoxidized soybean oil (AESO) green composite. J Adhes Sci Technol. 2013; 27: 1335-47.
- Islam MS, Saito JA, Emdad EM, Ahmed B, Islam MM, et al. Comparative genomics of two jute species and insight into fibre biogenesis. Nat Plants. 2017; 3: 16223.
- Ashraf MA, Zwawi M, Taqi Mehran M, Kanthasamy R, Bahadar A. Jute based bio and hybrid composites and their applications. Fibers. 2019; 7: 77.
- Liu R, Dong A, Fan X, Wang Q, Yu Y, Cavaco-Paulo A. HRP-mediated polyacrylamide graft modification of raw jute fabric. J Mol Cat B Enzym. 2015; 116: 29-38.
- Mondal MIH, Khan MMR, Islam MM, Islam MS, Rabbi MA. Characterization of grafted jute fiber using acrylate monomers pretreated with alkali. Fashion Text. 2016; 3: 9-14.
- Aly-Hassan MS. A new perspective in multifunctional composite materials. Multifunctionality Polym Compos. 2015: 42-67.
- Samanta AK, Chakraborty S, Guha Roy TK. Dyeing of jute with reactive dyes: optimisation of the process variables and assessment of colourfastness characteristics. J Inst Eng (India) Ser E. 2012; 93: 15-24.
- Roy AN, Samanta KK, Patra K. Physico-chemical properties of black yak fibre and its modification for blending with jute fibre. J Nat Fibers. 2017; 16: 1-12.
- Gon D, Das K, Paul P, Maity S. Jute composites as wood substitute. Int J Text Sci. 2013; 1: 84-93.
- Mondal MIH, Hoque MA, Latif MA, Islam MK. Improved characteristics of acetic anhydride and phthalic anhydride modified jute fibers. Res J Text Apparel. 2014; 18: 64-70.
- Samanta AK, Mukhopadhyay A, Ghosh SK. 2- processing of jute fibres and its applications. In: Kozlowski RZ, Mackiewicz-Talarczyk M, editors. Handbook of natural fibres. 2nd ed. UK: Woodhead Publishing; 2020; 49-120.
- Mondal IH. Grafting of methyl acrylate and methyl methacrylate onto Jute fiber: Physico-Chemical Characteristics of the Grafted Jute. J Engineered Fibers Fabr. 2013; 8: 42-50.
- Montazer M, Salehi A. Novel jute yarns grafted with methyl methacrylate. J Appl Polym Sci. 2008; 107: 2067-73.
- Gupta KC, Khandekar KK. Ceric(IV) ion-induced graft copolymerization of acrylamide and ethyl acrylate onto cellulose. Polym Int. 2006; 55: 139-50.
- Abdelmouleh M, Boufi S, Belgacem MN, Dufresne A, Gandini A. Modification of cellulose fibers with functionalized silanes: effect of the fiber treatment on the mechanical performances of cellulose-thermoset composites. J Appl Polym Sci. 2005; 98: 974-84.
- Ghosh P, Das D. Modification of cotton by acrylic acid (AA) in the presence of NaH2PO4 and K2S2O8 as catalysts under thermal treatment. Eur Polym J. 2000; 36: 2505-11.
- Mondal IH. Graft copolymerization of nitrile monomers onto sulfonated jute-cotton blended fabric. J Appl Polym Sci. 2003; 87: 2262-6.
- Mondal MIH, Farouqui FIF, Hanif MAH, Shafiur Rahman GMSR, Hoque MAH. Grafting of Methacrylonitrile and methylMethacrylate onto jute fibre: physico-chemical characteristics of grafted jute. J Appl Sci. 2005; 5: 1767-71.
- Misra M, Mohanty AK, Singh BC. A study on grafting of methyl methacrylate onto jute fiber (S2O82-thiourea redox system). J Appl Polym Sci. 1987; 33: 2809-19.
- Sharma BR, Kumar V, Soni PL. Graft copolymerization of acrylonitrile onto Cassia Tora gum with ceric ammonium nitrate-nitric acid as a redox initiator. J Appl Polym Sci. 2003; 90: 129-36.
- Samal RK, Samantaray HS, Samal RN. Graft copolymerization with a new class of acidic Peroxo salt IV. Grafting of acrylamide onto jute fiber using potassium monopersulphate: catalyzed by Fe(II). Polym J. 1986; 18: 471-8.
- Alam MS, Khan GMA, Razzaque SMA, Hossain MJ, Minhaz-ul-Haque M, Zebsyn S. Dyeing of cotton fabrics with reactive dyes and their physico-chemical properties. Indian J Fibre Text Res. 2008; 33: 58-65.
- Barquero A, Agirre A, Barandiaran MJ, Leiza JR. Monitoring the evolution of the microstructure of vinyl silane monomer containing poly(vinyl acetate) based copolymer latexes during storage. Eur Polym J. 2019; 121: 109299.
- Özmen N. A study of the effect of acetylation on hemp fibres with vinyl acetate. BioResources. 2012; 7: 3800-9.
- Tosh B, Routray CR. Grafting of cellulose based materials: a review. Chem Sci Rev Lett. 2014; 3: 74-92.
- Hong CK, Kim N, Kang SL, Nah C, Lee Y-S, et al. Mechanical properties of maleic anhydride treated jute fibre/polypropylene composites. Plast Rubber Compos. 2008; 37: 325-30.
- Cai Y, David SK, Pailthorpe MT. Dyeing of jute and jute/cotton blend fabrics with 2: 1 pre-metallised dyes. Dyes Pigments. 2000; 45: 161-8.
- Textile value chain, textile testing and quality control; 2020. Available from: https://textilevaluechain.in/in-depth-analysis/articles/textile-articles/textile-testing-and-quality-control/.