
Research Article
Adv Res Text Eng. 2023; 8(1): 1080.
Alkaline Hydrolysis of Polyester Fabric and Dyeing with Natural Colorants Extracted from Henna Leaves
Bayeleyegn Mekonnen Aragie; Asaye Dessie Wolela*
Department of Textile Engineering, Kombolcha Institute of Technology, Wollo University; Kombolcha, Ethiopia
*Corresponding author: Asaye Dessie Department of Textile Engineering, Kombolcha Institute of Technology, Wollo University, Ethiopia. Email: ase25dessie@gmail.com
Received: May 08, 2023 Accepted: June 06, 2023 Published: June 13, 2023
Abstract
The aim of this study was to dye polyester fabric with natural colorant obtained from henna leaves. Due to the hydrophobic nature of polyester, it makes the dyeing process difficult at low temperature and also prevents dye molecules from diffusing into the fiber interior during dyeing. To solve such dyeability problems of polyester, the fabric were treated with alkali to convert the hydrophobic nature into hydrophilic. This alkaline hydrolysis of polyester improved its chemical properties and made dyeing of polyester with natural colorant can be possible. The coloration of hydrolyzed polyester was carried out with the extracted aqueous natural dye. In this study optimizations of surface treatment conditions of polyester were investigated and the effects of hydrolysis on the fabric properties were also studied. Dyeing conditions like MLR, time, temperature and pH were optimized based on their dyeing properties results. Modifications of the fiber surface were confirmed by wettability test and FTIR study. The alkaline hydrolysis of polyester showed weight loss but achieved weight loss has no significant effect on the fabric’s strength. Beside this, characterization of the dyeing properties like color strength, dyeing evenness and color fastness were carried out for lawsone (primary colorant in henna leaves) dyed polyester. It was observed from the study that as the dyeing temperature and time increases, the K/S value increases until dye exhaustion attains equilibrium and then there is decrease. Higher color strength was obtained at basic pH conditions. Color fastness to washing and rubbing result obtained showed good to excellent grades.
Keywords: Polyester; Hydrolysis; Henna dye; Dyeing
Introduction
Natural dyes can provide not only a rich and varied source of dyestuff, but also an income through sustainable harvest and selling of these plants in many developing countries such as Ethiopia, Nigeria, Uganda, India, and Iran [1]. Natural dyes or colorants may be derived from several different parts of the plant kingdom, including leaves, fruits, wood, heartwood, roots, bulbs, seeds, and barks. Ethiopia is home to a plethora of plant species that produce dye [2]. Natural dyes are in high demand around the world these days, thanks to a growing understanding of their therapeutic properties [3].
Polyethylene Terephthalate (PET) is an aliphatic–aromatic polymer composed of Terephthalic Acid (TPA) and Ethylene Glycol (EG) that is the most commercially important and long- established polyester fiber material [4,5]. Polyethylene terephthalate macromolecules have large molecules and an inflexible structure, resulting in a compact and highly crystalline molecular arrangement in polyester fiber, which is correlated with a high melting point and glass transition temperature, as well as insensitivity to moisture and various chemicals [6].
Polyester fiber is hydrophobic in nature [7] due to its strongly crystalline structure and lack of polarity, which prevents water molecules from entering the polymer system and limits swelling in water. As a result, the hydrophobicity of polyester fibers prevents dye molecules from diffusing into the fiber interior during dyeing [8], making the dyeing process extremely difficult at low temperatures. Because of its small particle size and non-ionic nature, synthetic colorants such as disperse dye are used to dye polyester fiber at high temperatures and pressures [5].
However, due to rising global awareness and environmental concerns, there has been a renewed interest in natural dyes from various natural resources such as onion, madder, saffron, kola nut, dolu, and curcumin, which has led to several studies focusing on coloration of polyester fibers with natural dyes from various natural resources such as onion, madder, saffron, kola nut, dolu, and curcumin [1,9,10]. Several researches used the conventional exhaust approach to dye polyester fibers with natural dyes like henna [5]. However, no systematic study of henna dye application on alkali-treated polyester fabric has been published. As a result, the aim of this study was to see how Henna dyeing of alkali surface modified polyester with different metallic mordants like copper sulphate, aluminum sulphate, and iron sulphate, as well as bio mordants like lemon, affected the results. It is anticipated that mordanting with lemon would result in the introduction of tannins [11], which will develop binding properties that will improve the dyeing of polyester fabric.
The majority of textile factories use disperses dyes for polyester coloration, which are extremely polluting to the atmosphere. As a result, this study was conducted in order to ensure the commercial use of henna leaves and to introduce an environmentally sustainable dyeing method for polyester fabric [2].
This means that textiles can be treated to achieve hydrophilization or hydrophobization; additionally, the surface chemistry and topography can be affected to enhance adhesion and repellence properties, as well as the confinement of functional groups to the surface. The hydrophobic polyester was converted to the hydrophilic polyester in this study by treating it with alkali. Aqueous sodium hydroxide hydrolyzes polyester, which undergoes nucleophilic substitution. Polyester's alkaline hydrolysis offers hydrophilicity, wettability, and increased moisture resorption, among other benefits.
Polyethylene Terephthalate (PET) fibers have excellent chemical, physical, and mechanical properties, but their inherent hydrophobic and inert nature has some disadvantages. Low moisture regain and wettability cause a number of issues during manufacturing (static electricity build-up) and during consumers use (clinging to the body, accumulation of fluff and soil). Alkaline hydrolysis of polyester, which turns hydrophobic PET into hydrophilic due to fibre surface modification [12], can solve these problems.
Lawsonia inarmis produces “lawsone,” also known as hennotannic acid, a red orange dye molecule. This molecule has been used to dye skin, fingernails, hair, leather, silk, and wool because of its affinity for protein bonding [13,14]. Henna (L. inermis) is a plant that grows wild in abandoned areas. Henna is commonly used as a dyeing agent in the cosmetic industry, and it is used to color hair, skin, and nails [14].
Lawsone is the primary colorant in henna leaves, and its chemical formula is 2-hydroxy-1, 4-napthoquinone, as shown in Figure 1. Natural orange 6 (CI 75480) is a substantive keratin dye that imparts an orange color due to the inclusion of a –OH (auxochrome) group in the naphthoquinone structure [15].
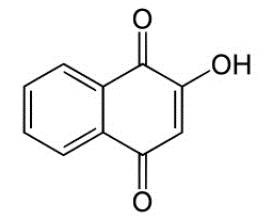
Figure 1: 2-Hydroxy-1, 4-Napthoquinone [16].

Figure 2: Collection and Preparation of henna plant leaves.
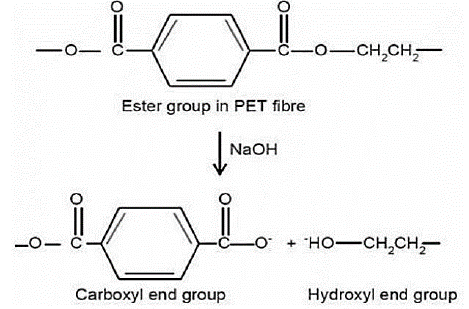
Figure 3: Alkali Hydrolysis of Polyester.
Natural dyes have a higher biodegradability and are therefore more environmentally friendly. Henna is non-toxic and does not irritate the skin [16,17].
The aim of this research is to use a colouring component obtained from henna leaves in combination with different types of metallic salt mordants and a bio-mordant to color hydrophilic polyester fabrics.
Experimental
Dyestuff
The natural dye derived from the henna plant leaf was used to dye the polyester. Henna, a natural dye, was used as a source of coloring. The leaves of the henna plant were collected in the surrounding areas of kombolcha district, Ethiopia.
Fabric
Pre-treated 100% polyester fabrics with specifications having GSM 86 g/m², twill weave 2x1, EPI=116, PPI=58 were used in this study.
Chemicals Used
Auxiliaries used dyeing process was also used in this study. Caustic soda was used for surface treatment of the polyester fabric. Laboratory grade sodium sulphate, copper sulphate, iron sulphate, aluminium sulphate, citric acid, standard detergents were used.
Equipment’s/Apparatus and Machineries
All the dyeing accessories used in dyeing process were also used in this study. The equipment’s and machineries used includes Centrifugal Grinding Machine, Lien Dyeing Machine, Data color 850 Spectrophotometer, Universal Tensile Strength Testing Machine, Launder-o-meter, Crock-meter, Shirley Stiffness Tester.
Collection and Preparations of Plant Material
Henna plant leaves were collected around the nearby areas of kombolcha town. The plant leaves were washed with water to remove the adhering materials and then dried by exposing to sunlight. The dried henna plant leaf was then crushed into fine powder for effective extraction. The powdered sample was used for further studies.
Dye Extraction
In order to carry out the dyeing of polyester with henna dye, the colorant was extracted from the finely powdered henna leaves. The henna leaf powder was dissolved in distilled water and allowed to boil in a beaker kept over water bath for quick extraction. The extractions of colorant were carried out at boil for 1hr, keeping material to liquor ratio 1:20. The extracted henna dye was then filtered through clean cotton cloth. The filtration was repeated two to three times, until there was no colorant left behind. The filtrate obtained is the extracted liquid dye and was used for dyeing.
Surface Modification of Polyester Fabric by Alkali Treatment
Polyester fabrics were treated with sodium hydroxide solution to alter the surface of polyester fibers and provide hydrophilicity. The treatments were carried out at different treatment conditions such as temperature (40, 50, 60, 70, 80 and 90°C), time (30, 45, 60, 75, 90, 105 and 120 minutes) and concentration of caustic soda (3%, 6%, 9% and 12% of o.w.f). Then the optimum treatment conditions were selected and the effects of hydrolysis on the fabric properties were studied.
Optimization of Dyeing Conditions for Polyester Dyeing
In order to dye alkali treated polyester fabric with henna leaf extract, optimization of the dyeing conditions is very important. Dyeing conditions like temperature, time, MLR and PH were optimized in the present study. For optimization of dyeing temperature, polyester dyeing was carried out at 80, 90, 100, 110, 120, 130, 140 and 150°C. For optimization of dyeing time, dyeing was carried out for 30, 45, 60, 75, 90, 105 and 120 minutes. To ascertain the optimum dyeing MLR, polyester samples were dyed at 1:5, 1:10, 1:15, 1:20, 1:25, 1:30 1:35 and 1:40 liquor ratio. Finally to optimize the dyeing pH, dyeing was carried out at pH of 4.5, 7, and 9.5.
Dyeing parameters were then optimized depending upon their dyeing properties results. Dyeing properties such as color strength values, dyeing evenness and color fastness were used to optimize the dyeing conditions.
Dyeing Procedures
The dyeing was performed by using 15% owf of dye concentrations. The alkali treated polyester fabric was dyed at 130 for 75 minutes at a material to liquor ratio of 1:25 for optimum dye exhaustion. The dyeing was performed in exhaust method in alkaline pH (pH 9-10). After dyeing, the dyed samples are rinsed with cold water and washed in a bath using 5g/L of soaping agent at 60°C for 10 minutes and then rinsed with cold water and finally dried in a dryer.
Mordanting
To study the effect of mordanting techniques in alkali treated polyester fabric dyeing with henna leaves extract; mordanting was carried out at pre-mordanting, post-mordanting and simultaneous mordanting methods. The mordant was applied at 80°C for 60 minutes with an MLR of 1:20. Copper sulphate, aluminium sulphate, iron sulphate, and citric acid mordants were used in this analysis. After that, color strength and fastness properties were measured and optimized to investigate the effect of mordanting techniques and mordant kind.
Evaluation of the Properties of the Surface Modified Polyester Fabric
Determination of Weight Loss: The weight (mass per unit area) of the fabric was tested according to ASTM D3776/ D3776M–20 standard test methods. The percentage weight loss of the alkali treated polyester fabric was calculated according to equation (1):
Weight loss (%) = [(W1 - W2)]/W1x100 (1)
Where, W1 and W2 are the weights of fabric before and after treatment, respectively
Determination of Tensile Strength: Tensile strength and extension at break was determined using universal tensile strength testing machine according to ASTM D2209-00(2015). The measurements were carried out for the treated and untreated samples in triplicate and average values of each were reported.
Determination of Stiffness: According to ASTM D1388 –18 standard test method the stiffness of the fabric was tested. Drapability was checked by measuring the bending length of the sample. Shirley stiffness tester was used for the bending length measurement.
Determination of Wettability:
Water Drop Absorbency: The water absorbency was measured as per AATCC TM79-2010e2 (2018)e standard. Water absorbency test was performed by putting a drop of water using dropper on the fabric surface and visually noting down time for the water absorption.
Sinking Time: Three pieces from each sample (2.5cmx2.5cm) were taken. The samples were carefully placed (each piece separately) in to glass beaker filled with water and time required for the sample to sink at the bottom of the beaker was noted. Average of three tests is reported.
Capillary Rise: Vertical wicking of textiles was tested according to AATCC TM197-2013 test methods. The hydrophilic properties were tested as follows: one end of the sample was immersed vertically along its length in a water reservoir and water diffused into the sample by capillary action. The height climbed by the water in the sample (the so called wicking height), was measured after 5 minutes. In this case each specimen was cut vertically into a 20x2cm strip, hung length way with the bottom end dipped in water stained with acid dye (for visibility).
Evaluation of the Properties of the Dyed Fabrics
Colour Strength: The color strength (K/S) of the dyed polyester fabric samples was measured using data color 850 Spectrophotometer by applying the Kubelka-Munk equation (2):
K/S = [(1-R) 2/ 2R] (2)
Where, K is the light absorption coefficient, S is the light scattering coefficient while R is the D65/10 light reflection.
Evaluation of Color Fastness: Color fastness to washing of the dyed fabric samples was evaluated as per the AATCC TM61- 2013e2 testing methods. Color fastness to rubbing of the dyed samples was tested as per AATCC TM8-2016e. Color fastness to light was tested according to AATCC TM16.3-2020.
Result and Discussion
Alkali Surface Hydrophilization of Polyester Fabric
A common modification method for producing a fabric with desirable qualities is to soak polyester fabric in alkali medium. Alkali treatment of polyester fabric may result in a higher-performing fabric. The findings show that alkali treatment with NaOH at the appropriate temperature and time can hydrolyze the polyester fiber surface, resulting in the fiber changing to a soft cloth with draping, and that it can also enhance fabric properties like fabric regain, water absorbency, and fabric pilling. The dyeing and resultant colour properties of alkali treated polyester would be expected to change as progressively more material is hydrolytically removed from the fiber surface. The current study aims to use this treatment to improve natural dyes' dye capacity on polyester cloth, and factors influencing this treatment were investigated in order to achieve better color strength of dyed samples while maintaining weight loss within the range of not affecting the treated samples' tensile strength.
Effect of treatment Temperature
By keeping all other parameters constant and varying only the treatment temperature, the effect of treatment temperature on fabric properties was investigated.
Figure 4 shows that as the temperature of the alkali treatment rises, the percentage weight loss of the fabric rises with it. Polyester samples were treated in sodium hydroxide solution to determine the appropriate temperature that achieves maximum color intensity of the after dyed samples, taking into account its effect on the rapidity of the treatment and, ultimately, on weight loss. At higher temperatures, there was a greater percentage of weight loss, which resulted in a decrease in fabric strength. However, the necessary hydrolysis of polyester fiber surface was achieved at the optimum temperature (80°C), resulting in less percentage weight loss.

Figure 4: Effect of treatment temperature on fabric weight loss.
Effect of Treatment Time
The alkali treatment method was carried out at different treatment durations while keeping other parameters constant to investigate the value of treatment period and its effect on the alkali treatment and thus the weight loss of polyester fabric. The alkali-treated polyester samples were also dyed with Henna extract under specific conditions, then washed and air-dried.
Figure 5 shows that as the treatment period increases, the weight loss of the fabric increases as well. As a result, the duration of the alkali treatment has a significant effect on the properties of the alkali-treated polyester fabric as well as its dyeing value. As polyester fabric is treated with alkali for a longer period of time, the polyester fabric's strength is reduced. At the optimal treatment period, the desired modification level was achieved (75 minutes). Treatment of polyester above the optimum time showed a significant drop in the strength of the fabric.
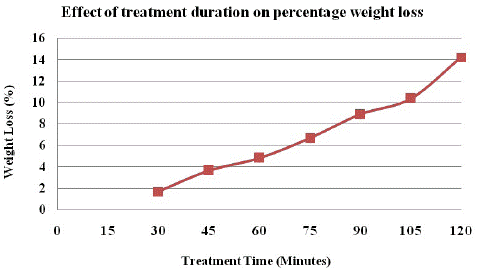
Figure 5: Effect of treatment time on polyester fabric weight loss.
Effect of Alkali Concentration
The effect of alkali concentration on the weight loss of polyester fabric was studied by varying the concentration of NaOH while keeping the treatment conditions constant.
It was observed from Figure 6 that as the concentration of alkali (NaOH) increases the percentage weight loss of the fabric increased. The concentration of alkali plays a significant role in the surface modification of polyester. A higher alkali concentration causes more weight loss, resulting in a decrease in fabric strength. However, in Figure 6, the necessary degree of hydrophilicity was achieved with a lower percentage of weight loss at the optimum alkali concentration (6%). The use of higher alkali concentration above the optimum level showed a significant reduction in fabric weight and strength.

Figure 6: Effect of alkali concentration on fabric weight loss.
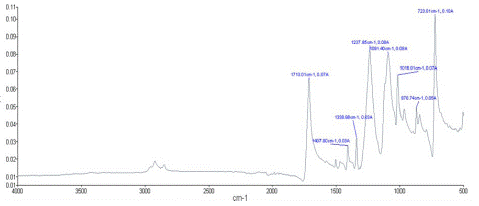
Figure 7: FTIR result of untreated polyester fabric.

Figure 8: FTIR result of alkali treated polyester fabric.
Effect of Hydrolysis on Weight Loss of Polyester Fabric
Almost all the studies on alkaline hydrolysis of polyester indicate weight loss. The amount of weight lost, however, is dependent on the hydrolytic conditions. The alkaline hydrolysis of polyester at the obtained optimum surface treatment conditions showed a loss in weight of the fabrics. It has been revealed that treating PET fabrics with alkaline causes the fabrics to lose weight. However, the weight loss value achieved in this study was within the normal acceptable range. That is to say, the weight loss achieved has no impact on the fabric's strength.
Effect of Alkali treatment on Wettability
The polymer structure and morphological structure of polyester fabrics decide its water absorbency. Since the polymer chains are broken due to the sodium hydroxide treatment, when the fabric's surface is changed, its absorbency increases but its strength decreases. Modification of fiber surface composition in textiles can change the entire surface wetting behaviour.
Wicking height versus wicking time indicates that there is an increase in wick ability due to the treatments. The result indicates a rise in wicking height in both the warp and weft directions. As compared to the untreated control polyester fabric, there is a 48.65% increase in warp direction and a 42.42% increase in weft direction. The results show that modified polyester has better wicking properties than regular polyester. This is due to the caustic soda hydrolysis of polyester.
The time it took for the samples to sink to the bottom of a beaker filled with water was used to calculate the sinking time. The NaOH-treated polyester samples sunk down in 18 seconds, while the untreated control polyester take long time.
The absorbency of sodium-hydroxide treated polyester fabric samples was determined by calculating water drop absorption time, which took less than 20 seconds for all samples tested in this study. Modified polyester absorbs more water than regular polyester. The factors which may contribute to this hydrophilicity are the increased surface roughness, the possible increase in the number of hydrophilic groups on the fiber surface caused by chain scisson and due to the increased accessibility of the available hydrophilic groups on the fiber surface owing to hydrolysis. Alkali treated polyester fabric samples showed remarkable increase of hydrophilicity. The time it takes for the polyester fabric to absorb water decreases as the concentration of caustic soda rises.
Confirmation of Hydrolysis of Polyester
The number of hydrophilic groups on the surface of polyester fibers increases as a result of hydrolysis. NaOH's nucleophilic attack on polyester chains triggers chain scissions at the ester linkages, resulting in carboxyl and hydroxyl polar groups, which increase polarity and hydrogen bonding capacity with water molecules, resulting in better water wet ability.
The current investigation confirmed that alkali treatment of polyester fabric results in fiber surface alteration. The treated polyester improved in water drop absorbency, sinking time, and wicking height, according to the wettability performance. The existence of new functional groups formed in the treated samples was also verified by the FTIR study. Furthermore, the alkali hydrolyzed polyester fabric dyed with henna dye performed better than the untreated polyester sample dyed with henna dye in terms of color strength. It means the treatment of polyester fabric with NaOH shown the modification of polyester fiber surface and had a significant effect in the dyeability and property of polyester fabric.
FTIR Analysis
FTIR measurements have been carried out using an infrared spectroscopy to study the availability of new functional groups with a test method ASTM D629-99.
Effect of Alkali treatment on Fabric Physical Property
The tensile strength of the fabric were tested as per ASTM D2209 - 00(2015) testing methods. Average value of each test was reported in this study.
It is evident from Table 1 that alkali treatment fabric showed 5.3% drop in tensile strength in warp direction and 6.44% in weft direction. The obtained drop in tensile strength is within the standard acceptance. Even though there is a debate among the researchers, the acceptable limit of tensile strength loss is from 5-10%. This means the treatment of polyester with alkali at the optimum treatment conditions don’t affect the strength of fabric.
Tensile Strength (g/tex)
Bending Length (cm)
Test Direction
Warp
Weft
Warp
Weft
Before treatment
34.32
32.3
2.9
2.6
After treatment
32.5
30.22
1.6
1.5
Table 1: Effect of alkali treatment on physical property.
Mordants
Washing Fastness
Rubbing Fastness
Color change
Staining
Dry
Wet
Copper Sulphate
5
5
4-5
4-5
Iron Sulphate
5
5
5
4-5
Aluminium Sulphate
5
5
5
4-5
Citric Acid
5
5
4-5
4-5
Table 2: Effect of pre-mordanting on fastness properties.
Mordants
Washing Fastness
Rubbing Fastness
Color change
Staining
Dry
Wet
Copper Sulphate
5
5
4-5
4
Iron Sulphate
5
5
4-5
4
Aluminium Sulphate
5
5
5
4-5
Citric Acid
5
5
4-5
4
Table 3: Effect of post-mordanting on fastness properties.
Mordants
Washing Fastness
Rubbing Fastness
Color change
Staining
Dry
Wet
Copper Sulphate
5
5
4-5
4-5
Iron Sulphate
5
5
5
4-5
Aluminium Sulphate
5
5
5
5
Citric Acid
5
5
5
4-5
Table 4: Effect of simultaneous mordanting on fastness properties.
The stiffness or drapability of a fabric can be determined by its bending length. There is a decrease in the bending length of the fabric due to weight reduction process. As compared to the untreated control polyester, the handled polyester's bending length decreases by 44.83 % in the warp and 42.31% in the weft directions. This demonstrates that the untreated fabric is stiffer than the one that has been treated. The treated polyester fabric samples drapes well. This occurs as a result of pits or voids forming on the fabric's surface. Furthermore, the reduced diameter of fibers and thickness of fabric can cause a decrease in bending length of treated fabric.
Effect of Dyeing Conditions on Dyeability of Polyester with Henna Dye
Effect of Dyeing Temperature
At different temperature levels, the effect of temperature on the dyeability of polyester fabric with henna dye extract was investigated, and the color strength of the dyed fabric was measured.
It can be seen in Figure 9 that the color intensity increases with increasing dyeing temperature and reaches a maximum value at 130°C dyeing temperature. Furthermore high temperatures caused lower colour strength and unevenness as well. This increase in dye uptake can be explained by fabric swelling and de aggregation of dye molecules into single molecule at higher temperatures.
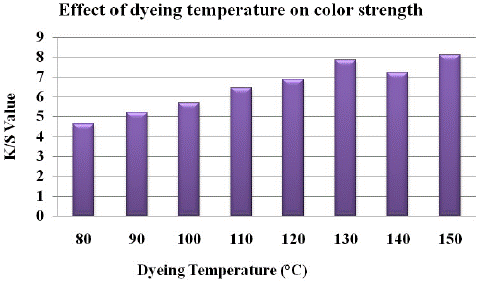
Figure 9: Effect of dyeing temperature on K/S value.
In this study it was revealed that as dyeing temperature increases the K/S value increases until dye exhaustion attains equilibrium and then there is decrease in the colour strength after further increase in temperature from 130. This effect was observed due to shift in equilibrium of colorant from fabric to dye bath. Then again the color strength value starts to increase with further increase in dyeing temperature after the equilibrium point.
Effect of Dyeing Time
The effects of dyeing time on the dyeability of alkali modified polyester fabric dyed with the extracted henna dye were investigated by measuring the dyeing properties of the dyed polyester sample.
Figure 10 shows that the longer the dyeing time, the higher is the colour strength until dye exhaustion attains equilibrium and there is decrease in the colour strength after further increase in time from 75 minutes. This effect was observed due to shift in equilibrium of colorant from fabric to dye bath.
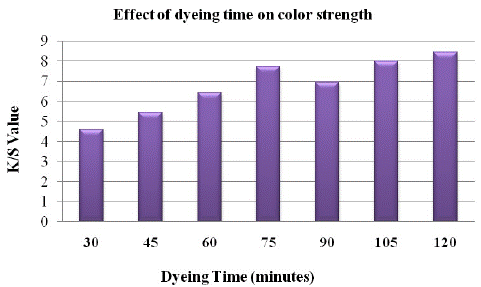
Figure 10: Effect of dyeing time on K/S value.
Effect of Dyeing pH
To study the effect of dyeing pH on the dyeability of polyester fabric dyed with natural dye obtained from Henna plant leaves, the dyeing of polyester was carried out at acidic, neutral and basic media.
It was observed from above Figure 11 that the best color strength is obtained at basic pH. The pH value of the dye bath has considerable effect on the dye ability of polyester fabrics while using Henna’s aqueous extract.
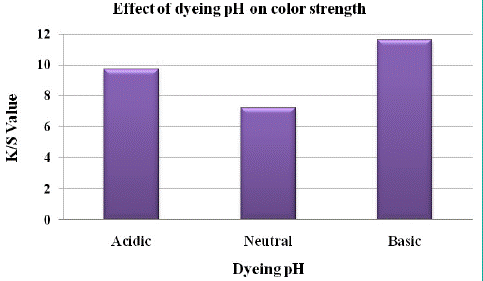
Figure 11: Effect of dyeing pH on K/S value.
Effect of Dyeing MLR
It is clear that colour strength increases with increase ML ratio and the optimum ML ratio was 1:25 as it was indicated in Figure 12. Lower relative colour strength value with low ML ratio could be explained that with the congestion of dye molecules at lower ML ratio which compelled dye molecules to strike each other rather than with fabric for bond formation.

Figure 12: Effect of dyeing MLR on K/S value.
Fastness Properties of Dyed Fabric
The henna extract natural dyed polyester fabric was mordanted with pre-mordanting, post- mordanting and simultaneous mordanting techniques and the one which gives higher result was preferred. Copper sulphate, iron sulphate, aluminium sulphate and citric acid were used as a mordant in each techniques of mordanting methods in the present study. The mordant which provided better result was preferred.
Effect of Pre-Mordanting
In the pre-mordanting process, the polyester fabric dyed with natural dye mordanted with all the types of mordant used in this study showed excellent washing fastness properties. That means, there are no color change and staining at all observed in the dyed polyester. The rubbing fastness of the dyed sample showed good to excellent score.
Effect of Post-Mordanting
As the effect of post-mordanting methods on the fastness properties of henna extract dyed polyester fabric was studied, the washing fastness was assessed in terms of color change and staining. It was observed that there is no color change and staining in all types of mordant used. Good to excellent in the dry rubbing fastness and good grade in the wet rubbing fastness was obtained. However, one grade down result was obtained in the wet rubbing fastness as compared to the pre-mordanting.
Effect of Simultaneous Mordanting
In average better fastness properties result was obtained in the simultaneous mordanting method as compared to the other mordanting methods. Excellent washing fastness grade and good to excellent rubbing fastness was obtained. It can be concluded that simultaneous mordanting preferred for dyeing of polyester with natural dye obtained from henna leaves. And also, in average the aluminium sulphate mordant given better fastness grade in all methods of mordanting and can be preferred for polyester dyeing with henna.
Conclusion
The majority of textile factories use disperses dyes for polyester coloration, which are extremely polluting to the atmosphere. However, in this investigation an attempt was made to dye polyester fabric with natural dyes extracted from henna leaves which is a new approach in textile coloration. It was observed that there is difficulty in dyeing of unmodified polyester with lawsone dye due to the hydrophobic and inert nature of the polyester fabric. As a result, surface alterations of the polyester fabric were carried out by treatment with alkali to convert hydrophobic into hydrophilic nature in order to improve the dyeability properties of polyester. Alkaline hydrolysed polyester was then colored with aqueous extract of henna dye at high temperature and the properties of the dyed fabric were measured. Better color strength value, washing and rubbing fastness was achieved. However, a slight drop in weight loss was observed but which is in the acceptable range and doesn’t affect tensile strength of the treated samples. The effect of metallic mordant and bio-mordant were studied and observed comparable fastness results. In average the simultaneous mordanting showed better K/S and fastness properties in various mordant types. The research findings therefore suggest that henna dye can be used in coloration of polyester at high temperature.
Author Statements
Acknowledgements
The authors would like to thank to Kombolcha Textile Share Company (KTSC) for supporting us to characterize all the samples. And also to Kombolcha Institute of Technology (KiOT) for supporting us to conduct the laboratory works.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Ado A, Musa H, Gumel SM, Yahaya H. Eco-friendly dyeing of cotton and polyester fabrics with natural dyes extracted from different varieties of Kola nuts. International Journal of Chemical Sciences. 2015; 1: 6-11.
- Al Mamun MA, Hossain MM, Khan MA. Dyeing of Polyester Fabric with Natural Colorants Extracted from Mahogany (Swieteniamahagoni) Seed Pods. Journal of Engineering Science. 2020; 11: 37-42.
- Hasan M, Abu Nayem K, Anwarul Azim AYM, Ghosh NC. Application of purified Lawsone as natural dye on cotton and silk fabric. Journal of Textiles. 2015.
- Deopura BL, Alagirusamy R, Joshi M, Gupta B. Polyesters and polyamides. Elsevier. 2008.
- Bhuiyan MR, Ali A, Islam A, Hannan MA, Kabir SF, et al. Coloration of polyester fiber with natural dye henna (Lawsonia inermis L.) without using mordant: a new approach towards a cleaner production. Fashion and Textiles. 2018; 5: 1-11.
- Lewin M, Pearce EM. Handbook of fiber chemistry. Boca Raton: CRC. 2007.
- El-Nagar K, Saudy MA, Eatah AI, Masoud MM. DC pseudo plasma discharge treatment of polyester textile surface for disperses dyeing. Journal of the Textile Institute. 2006; 97: 111-117.
- PETERS RH, INGAMELLS W. Theoretical aspects of the role of fibre structure in dyeing. Journal of the Society of Dyers and Colourists. 1973; 89: 397-405.
- Elnagar K, Abou Elmaaty T, Raouf S. Dyeing of polyester and polyamide synthetic fabrics with natural dyes using ecofriendly technique. Journal of Textiles. 2014.
- Shahin MF, Ahmed RM, Marie MM. Optimizing the dyeing process of alkali-treated polyester fabric with dolu natural dye. International Journal of Engineering Research and Applications. 2014; 4: 35-40.
- Ezeabara CA, Okeke CU, Ilodibia CV, Aziagba BO. Determination of tannin content in various parts of six citrus species. Journal of Scientific Research and Reports. 2014; 1384-1392.
- Getnet M, Chavan R. Catalyzation of alkaline hydrolysis of polyester by oxidizing agents for surface modification. Int J Sci Basic Appl Res. 2022; 232.
- Singh YV, Kumar S, Singh M. Agro History, Uses, Ecology and Distribution of Henna (Lawsonia Inermis) Henna Cultivation, Improvement and Trade Jodpur, India. Central and Arid zone Research Institute. 2005; 11-12.
- Chukwu OOC, Odu CE, Chukwu DI, Hafiz N, Chidozie VN, et al. Application of extracts of henna (Lawsonia inamis) leaves as a counter stain. 2011; 5: 3351-3356.
- Mohammad F, Yusuf M, Shahid M, Khan SA, Khan MI, et al. Dyeing of wool with the extract of henna leaves using mixed metal mordants. Colourage. 2012; 59: 51-57.
- Nataraj P, Selvan T, Kumaran S. DYEING OF SILK WITH LAWSONIA INERMIS [HENNA] EXTRACT AND STUDYON THEIR FASTNESS PROPERTIES. International Journal of Research-GRANTHAALAYAH. 2016; 4: 101-106.
- Alam MM, Rahman ML, Haque MZ. Extraction of henna leaf dye and its dyeing effects on textile fibre. Bangladesh Journal of Scientific and Industrial Research. 2007; 42: 217-222.