Special Article: Minimally Invasive Surgery
Austin J Surg. 2023; 10(5): 1315.
A Case Series and Literature Review of SGLT2 Inhibitors and Fournier’s Gangrene: an Overlooked Rare Interaction
Ahmad Saadya MBBCh, MRCS¹; Miss Eleni Hadjikyriacou, MBBCh, MRCS²
1Queen Victoria Hospital, East Grinstead, UK
2Morriston Hospital, United Kingdom
*Corresponding author: Ahmad Saadya Department of Plastic and reconstructive surgery, Queen Victoria Hospital, Holtye Rd, East Grinstead RH19 3DZ, UK Tel: +447846601739 Email: a.saadya@nhs.net
Received: September 25, 2023 Accepted: October 21, 2023 Published: October 28, 2023
Abstract
Introduction: Fournier’s Gangrene (FG) is a rare but life-threatening genital infection that can occur in vulnerable patients, especially diabetic patients. Literature evidence is suggesting that novel diabetic medication Sodium-glucose Cotransporter-2 (SGLT2) Inhibitors can cause Fournier’s gangrene.
Patients and Methods: We report three cases of patients with Fournier’s gangrene we have treated who have been using the novel Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors for the treatment of type II diabetes mellitus and our experience treating them. We also inspect the current literature looking into the incidence of this rare event and the link to this class of medications.
We also have done a brief review of 24 articles including published case reports, pharmacovigilance studies, systematic reviews and meta-analysis, retrospective and cohort studies was done. Literature review showed an obvious link between SGLT2 inhibitors and FG.
Conclusion: The rising number of FG cases since the introduction Sodium-glucose Cotransporter-2 (SGLT2) Inhibitors should be alarming to the prescribing physician and patients should be evaluated carefully for their risk factors for Fournier’s gangrene. Surgeons should suspect the incidence of Fournier’s gangrene in any patient presenting with signs of sepsis and signs of genital infection and using Sodium-glucose Cotransporter-2 (SGLT2) Inhibitors. Patient education about the risk is needed to initiate prompt diagnosis and treatment. After life-saving measures like surgical debridement and antibiotics, the SGLT2 inhibitor agent should be discontinued and alternative therapy for glycaemic control should be provided.
Abbreviations: SGLT2: Sodium-glucose Cotransporter-2; FG: Fournier’s Gangrene; Reconstruction
Introduction
In 2018, the US Food and Drug Administration (FDA) issued a black box warning about multiple case reports of Fournier’s gangrene that were observed in patients using Sodium-Glucose cotransporter 2 (SGLT2) inhibitors. The UK Medicines and Healthcare products Regulatory Agency (MHRA) in 2019 has also issued a warning regarding the possible link between the use of Sodium-glucose cotransporter 2 (SGLT2) inhibitors and the developing of Fournier’s gangrene. Dapagliflozin and canagliflozin are Sodium-Glucose cotransporter 2 (SGLT2) inhibitors used in the treatment of type II diabetes mellitus. Fournier’s gangrene is a type of necrotizing fasciitis that happens in the urogenital area.
Materials/Patients
We report three cases of patients with Fournier’s gangrene we have treated who have been using the novel Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors for the treatment of type II diabetes mellitus and our experience treating them. We also inspect the current literature looking into the incidence of this rare event and the link to this class of medications.
We also have done a brief review of 24 articles including published case reports, pharmacovigilance studies, systematic reviews and meta-analysis, retrospective and cohort studies was done. Literature review showed an obvious link between SGLT2 inhibitors and FG.
Discussion
Despite being a rare condition, Fournier’s Gangrene (FG) remains one of the most dangerous life-threatening infections. Fournier’s Gangrene (FG) is a special type of necrotizing fasciitis infection that happens in the groin and perineal area. With high chances of mortality, prompt diagnosis and surgical intervention are crucial, and it is combined with different treatment modalities like antibiotics and multiple organ support to achieve good recovery. Fournier’s Gangrene (FG) susceptible population includes diabetic patients and immunosuppressed individuals with other risk factors like male gender, alcoholism, and hypertension.
Sodium-Gucose cotransporter 2 (SGLT2) inhibitors are orally administered antidiabetic drugs. They act by inhibiting the reabsorption of glucose from the urine by binding to SGLT2 in the proximal tubules of the kidney. This class of medications has been widely used in the past decade with impressive results. Studies showed a significant reduction in HBA1c, increased insulin sensitivity with a reduction of cardiac preload, and improving renal and hepatic functions. Sodium-Glucose cotransporter 2 (SGLT2) inhibitors also carry a notable multiple-risk profile that includes but not limited to diabetic ketoacidosis, urogenital infections, and acute kidney injury [1].
In 2018, the US Food and Drug Administration (FDA) issued a warning about multiple case reports of Fournier’s gangrene that were observed in patients using Sodium-Glucose cotransporter 2 (SGLT2) inhibitors [2]. The UK Medicines and Healthcare products Regulatory Agency (MHRA) in 2019 has also issued a warning regarding the possible link between the use of Sodium-glucose cotransporter 2 (SGLT2) inhibitors and the developing of Fournier’s gangrene [3].
Case Series
Case 1
A 61-year-old male patient presented with signs of septic shock to the emergency department. The patient’s medical history was significant for type II diabetes mellitus treated with Dapagliflozin and Metformin and hypertension treated with Amlodipine, Atenolol, Lisinopril, Doxazocin and Spironolactone and hypercholesteremia treated with atorvastatin and he was on a lifelong clopidogrel after having a cerebrovascular accident. The patient has been taking dapagliflozin for 11 months and has been diabetic for 10 years. On examination, patient had an abscess in the right groin, hemi-scrotum, and buttock with a wide area of necrotic tissue and foul-smelling discharge.
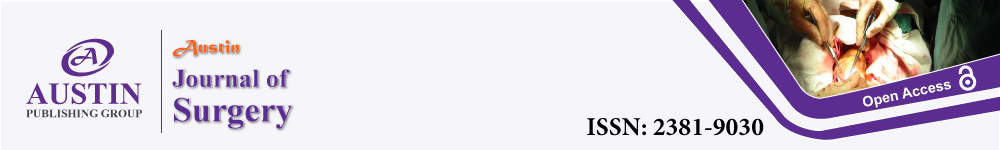
He was admitted for emergency surgery and debridement. The procedures included incision, drainage, and debridement of the right groin, hemi-scrotum, and buttock abscess with end colostomy, and he was transferred to intensive care unit post-operatively (Figure 1). The patient was treated with IV antibiotics (Meropenem and Clindamycin) and had a 2nd surgery 3 days later for a 2nd look and debridement and was subsequently moved to a ward care level 6 days after the 2nd look.
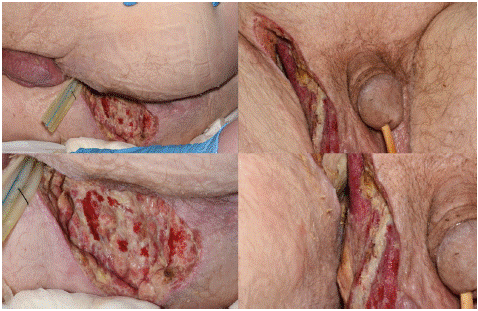
Figure 1: An image of the groin and scrotum on postoperative day 4 after debridement of Fournier’s gangrene.
At presentation, the patient had raised infection markers with a white blood cell count of 23.6 x 109 L and neutrophil count of 20.1x109 L. CRP of 225 mg/L (Figure 2). He also had deranged kidney function with urea of 21.7mmol/L and Creatinine of 162umol/L. Urinalysis was positive for white blood cells, red blood cells, and epithelial cells. Pre-operative microbiology swab results showed scanty growth of Hafnia Alvei (HALV), moderate growth of Mixed Anaerobes (MANA) and scanty growth of Actinomyces Neuii (ANEU). Intraoperative tissue biopsy samples showed light growth of Escherichia Coli (ECOL 2), Light growth of Proteus mirabilis (PMIR), Heavy growth of Fusobacterium Gonidiaformans (FGON) and Heavy growth of Porphyromonas Somerae.
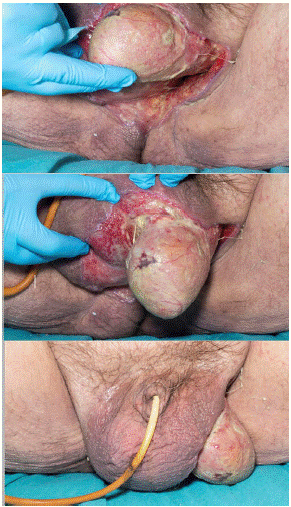
Figure 2: An image of the groin and scrotum on postoperative day 6 after debridement of Fournier’s gangrene.
During admission patient received treatment with intravenous antibiotics that were discontinued on day 14 of admission. He also received ongoing stoma care from stoma team. After stabilization and diabetic control, patient was transferred to a specialized burns unit for further treatment and surgical reconstruction of defects and was discharged 7 days post reconstruction.
Case 2
A 75-year-old male, diabetic and treated with Canagliflozin presented with a 4-day history of a painful perineal lump, signs of sepsis with diffuse scrotal swelling and erythema. A diagnosis with Fournier’s gangrene was established. On examination, he had a swollen red scrotum and necrotic grey offensive tissue at the inferior aspect to left hemi-scrotum with pockets extending posteriorly behind the left testis. He had a complex medical background of AF for which he was taking warfarin, type II diabetes mellitus, for which he was on Canagliflozin 150mg OD for 12 months prior to his presentation, hypertension, prostate cancer, chronic kidney disease stage 4 and high body mass index and he was an ex-smoker.
CT imaging showed fluid around the testes bilaterally and oedema of the scrotum with subcutaneous gas within the perineum and posteroinferior scrotum extending over an area of approximately 7x2x7.1cm consistent with necrotising fasciitis. The wound swabs grow mixed anaerobes and his WCC was 19x109L, CRP 236mg/L, and lactate of 3.2mmol/L.
The patient had immediate debridement under general anaesthetic and A 14 Fr catheter was placed (Figure 3).
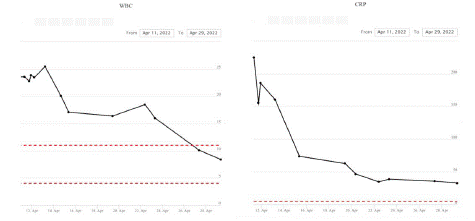
Figure 3: Blood test results over the duration of stay at the hospital showing results for White blood cell count and C-Reactive protein.
The canagliflozin was immediately stopped and changed to metformin, with the guidance and regular checks of the diabetic team.
The patient was finally transferred to burns unit for reconstruction 3 weeks after his admission to his local hospital and following 3 debridement procedures. The patient remained in our wards for 7 days prior to receiving SSG for wound optimisation, daily change of dressings with betadine-soaked gauze and showers. Catheter remained for 2 weeks to avoid contamination of the graft. The patient had an uneventful recovery during his admission in the Burns unit. He was discharged 3 days following his operation. He had outpatient follow up with our unit and eventually he required no further dressings.
Case 3
A 75-year-old male, diabetic and treated with empagliflozin presented with a 8-day history of a painful perineal lump presented to hospital. A diagnosis with Fournier’s gangrene was established. On examination, he had a swollen red scrotal abscess and necrotic tissue was noted at his right hemi-scrotum. Patient had a background of Type 2 diabetes which was being treated with metformin started 5 years prior to presentation and empagliflozin that was started 9 months prior to presentation. Patient also had a background of COPD, hypertension, previous PCI and previous carotid endarterectomy.
Blood tests showed raised CRP of 53mg/L, WCC 14x109L, The patient was admitted for emergency debridement under general anaesthetic and had a 2nd look and further debridement four days later with and had defunctioning colostomy 2 days after 2nd debridement. Patient was also started on intravenous antibiotics The empagliflozin was stopped and with the guidance and regular follow-up of the diabetic team. The patient was finally transferred to burns unit for reconstruction 2 weeks after his admission. The patient remained under the care of burns team after reconstruction for 3 days prior to receiving a split thickness skin graft for wound reconstruction. Daily change of dressings with betadine-soaked gauze and showers. Catheter remained for 1 week to avoid contamination of the graft. The patient had an uneventful recovery and received ongoing stoma care, and he was discharged 10 days following his reconstruction.
Literature Review
Despite being revolutionary in the treatment of diabetes with very good cardioprotective and safe renal profiles, there have been concerns regarding the role played by SGLT2 inhibitors in genital and perineal infections in patients using the treatment. We believe there is strong evidence in the literature supporting the link between FG and SGLT2 inhibitors. We have identified at least 9 published case reports for FG in patients taking SGLT2 for the treatment of diabetes. Additionally, we have also found multiple cohort and systematic analysis studies with similar conclusions.
Nagano et al reported a case of a 34-year-old male who was diagnosed with FG 142 days after starting treatment with empagliflozin for type II diabetes mellitus. The clinical examination, laboratory data, and CT findings were consistent with FG. Glycaemic control was good at the time of FG onset and the patient had no complications of diabetes before the onset of FG. Surgical debridement of necrotic tissues was performed immediately after admission to hospital and the patient’s wounds healed with no mortality.
In their study, Rodler et al [5] presented a case of a 39-year-old man who had type II diabetes and was taking oral dapagliflozin, metformin, and sitagliptin medications. The patient was admitted to the hospital with FG and underwent emergency scrotal surgery. However, due to respiratory and circulatory problems, he had to be transferred to the intensive care unit. After spending 27 days in the hospital with delirium, blood glucose fluctuations, and undergoing five more surgical interventions, the patient's condition finally stabilized, and he was discharged.
Two patients were described by Elbiddini et al. [6], the first being a 71-year-old female who had type II diabetes and was taking dapagliflozin medication. She presented with a large abscess in the perianal area, accompanied by a foul-smelling discharge. Her advanced age, obesity, diabetes, and site trauma placed her at risk for FG. The patient's treatment plan included discontinuing dapagliflozin and administering procedural debridement, wound care, and broad-spectrum intravenous antibiotics during her hospitalization.
The second patient was a 72-year-old man with type II diabetes who developed FG while taking canagliflozin medication. In addition to his diabetes and use of canagliflozin, his age, gender put him at risk for the condition. The patient received multiple surgical debridement procedures and broad-spectrum antibiotics as part of his treatment plan. He remained in the hospital for one month before being discharged with instructions for outpatient wound care and vacuum dressing changes. Canagliflozin was also discontinued during his hospital stay [7].
In their study, Kumar, and colleagues [8] presented another case of a 41-year-old man who had type II diabetes and was being treated with empagliflozin and metformin medications. The patient presented with scrotal swelling, and further tests revealed that he had developed Fournier's gangrene. He underwent emergency exploration and debridement followed by another exploration, washout, and application of a vacuum dressing in a later operation. Subsequently, he received a split skin graft to his perineum. The patient required a 2-week course of intravenous antibiotics and was later discharged home with oral antibiotics. During admission, the patient's empagliflozin medication was stopped, and he was started on a basal bolus insulin regimen to better manage his blood sugar levels.
Elshimy et al. [9] described a case of a male patient who developed Fournier's gangrene 10 days after beginning treatment with Empagliflozin. The patient had been diagnosed with type II diabetes mellitus approximately 10 years earlier and had peripheral neuropathy, Hashimoto's hypothyroidism, and morbid obesity, but no prior history of genital or urinary tract infections.
Ellegård et al. [10] reported a similar case in a diabetic female patient who had a past medical history of type II diabetes mellitus, for which she had been taking the SGLT-2 inhibitor dapagliflozin for 1,5 years. The patient also had other risk factors like obesity and immunosuppression.
It is also worth noting that another major side effect of SGLT2 inhibitors is diabetic ketoacidosis which has been reported in conjugation with FG in at least two cases [11,12].
The Emerging Evidence
According to Bersoff-Matcha et al. [13] in addition to published cases, the FDA has identified at least 55 unique cases of FG in patients receiving SGLT2 inhibitors between 1 March 2013 and 31 January 2019. Out of the 55 cases,16 cases were linked to dapagliflozin,18 were linked to empagliflozin and 21 were linked to canagliflozin; in comparison,19 cases of Fournier gangrene were identified for other antihyperglycemic agents between 1984 and January 2019. Interestingly 16 of the 55 cases were females [14] which is a rarity as the reported male-to-female ratio is 10-1 [15,16].
A pharmacovigilance study from 2013 to 2021 measuring the safety of SGLT2 inhibitors showed Fournier’s gangrene as one of the strong safety signals after examining more than 57000 records related to SGLT2i [17] and another pharmacovigilance study with data from the U.S. FDA Adverse Event Reporting System (FAERS) that has examined 542 FG cases in patients receiving SGLT2 inhibitors identified signals between the drug and FG [18].
A study from Korea based on the records the of National Health Insurance Service (NHIS) database that covered almost 98% of the total population in Korea investigated the association between SGLT2 inhibitors as an add-on therapy to metformin and the risk of FG incidence. The study included a total of 107131 patients and showed that 46 and 71 cases of genital and urinary infections were reported to the Korea Adverse Event Reporting System (KAERS) between 2016 and 2020 with the administration of SGLT-2 inhibitors as the suspected cause. In contrast, 0 and 18 reports were made for other second-line antidiabetic drugs Dipeptidyl Peptidase-4 (DPP-4) Inhibitors, Sulphonylureas (SU), and Thiazolidinediones (TDZ) during the same time, respectively [19].
This was also reported in another cohort retrospective study from the United States showing an increase in risk that was evident in the first month of SGLT2 treatment initiation and remained elevated throughout the course of therapy and SGLT2 inhibitors were associated with an approximately three-fold increase in the risk of genital infections[20]
An Australian cohort study examining 1977 patients showed that like existing evidence, they found a higher risk of genital infection associated with SGLT2 inhibitors (primarily dapagliflozin) [21].
A systematic review and meta-analysis of 77 studies suggested that the current evidence confirmed that SGLT2 inhibitors increase the risk of genital infections, and suggested that the effects on genital infections may differ among SGLT2 inhibitors [22] and is also worth mentioning that changes in the vaginal flora have been reported with using SGLT2i Luseogliflozin as an adverse effect [23].
While it is logical to assume that all diabetic patients can be at risk of having genital infections especially if they have other risk factors like obesity and hypertension, but it is also safe to say that there is overwhelming evidence showing a link between the genital infections and the use of SGLT2i [24]. A very specific patient education is needed to help prompt diagnosis and treatment of FG.
Conclusion
The rising number of FG cases since the introduction Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors should be alarming to the prescribing physician. Patients should be evaluated carefully for their risk factors for Fournier’s gangrene. Surgeons should suspect the diagnosis of Fournier’s gangrene in any patient presenting with signs of sepsis and signs of genital/perineal infection and using Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors. Patient education about the risk is needed to initiate prompt diagnosis and treatment. After the life saving measures like surgical debridement and antibiotics, The SGLT2 inhibitor agent should be discontinued and alternative therapy for glycaemic control should be provided. More studies should investigate the mechanisms of SGLT2 inhibitors causing Fournier’s gangrene.
References
- Xu B, Li S, Kang B, Zhou J. The current role of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus management. Cardiovasc Diabetol. 2022; 21: 83.
- FDA warns about rare occurrences of a serious infection of the genital area with SGLT2 inhibitors for diabetes | FDA [cited Dec 06, 2022]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-rare-occurrences-serious-infection-genital-area-sglt2-inhibitors-diabetes.
- SGLT2 inhibitors: reports of Fournier’s gangrene (necrotising fasciitis of the genitalia or perineum) – GOV.UK [cited Dec 06, 2022]. Available from: https://www.gov.uk/drug-safety-update/sglt2-inhibitors-reports-of-fournier-s-gangrene-necrotising-fasciitis-of-the-genitalia-or-perineum.
- Nagano Y, Yakame NK, Aoki H, Yamakawa T, Kondo NI. Fournier’s gangrene in a patient with type 2 diabetes mellitus treated with empagliflozin: A case report. Drug Saf Case Rep. Dec 2019; 6: 11.
- Rodler S, Weig T, Finkenzeller C, Stief C, Staehler M. Fournier’s gangrene under sodium-glucose cotransporter 2 inhibitor therapy as a life-threatening adverse event: A case report and review of the literature. Cureus. 2019; 11: e5778.
- Elbeddini A, Gallinger J, Davey M, Brassard S, Gazarin M, Plourde F et al. A case of Fournier’s gangrene in a patient taking canagliflozin for the treatment of type II diabetes mellitus. Am J Case Rep. 2020; 21: e920115.
- Elbeddini A, Tayefehchamani Y, Davey M, Gallinger J, Hooda N, Aly A, et al. Fournier’s gangrene with dapagliflozin in a rural hospital: a case report. BMJ Case Rep. 2021; 14: 237784.
- Kumar S, Costello AJ, Colman PG. Fournier’s gangrene in a man on empagliflozin for treatment of Type 2 diabetes. Diabet Med. 2017; 34: 1646-8.
- Elshimy G, Correa R, Alsayed M, Jyothinagaram S. Early presentation of a rare complication of sodium-glucose Cotransporter-2 inhibitors 10 days after initiation: case report and literature review. Cureus. 2019; 11: e5173.
- Ellegård L, Prytz M. Fournier’s gangrene under SGLT-2 inhibitor therapy: A literature review and case report. Int J Surg Case Rep. 2020; 77: 692-4.
- Kasbawala K, Stamatiades GA, Majumdar SK. Fournier’s gangrene and diabetic ketoacidosis associated with sodium glucose co-transporter 2 (SGLT2) inhibitors: life-threatening complications. Am J Case Rep. 2020; 21: e921536.
- Lindsay PJ, Gibson LE, Bittner EA, Berg S, Chang MG. Sodium-Glucose cotransporter-2 (SGLT2) inhibitor-induced euglycemic diabetic ketoacidosis complicating the perioperative management of a patient with type 2 diabetes mellitus (T2DM) and Fournier’s gangrene: A case report. Int J Surg Case Rep. Jan 2020; 77: 463-6.
- Bersoff-Matcha SJ, Chamberlain C, Cao C, Kortepeter C, Chong WH. Fournier gangrene associated with sodium-glucose Cotransporter-2 inhibitors: a review of spontaneous postmarketing cases. Ann Intern Med. 2019; 170: 764-9.
- McGill JB, Subramanian S. Safety of sodium-glucose co-transporter 2 inhibitors. Am J Cardiol. Dec 2019; 124: S45-52.
- Chennamsetty A, Khourdaji I, Burks F, Killinger KA. Contemporary diagnosis and management of Fournier’s gangrene. Ther Adv Urol. 2015; 7: 203-15.
- Prokhorov AV. Fournier’s gangrene. Kazan Med J. 2016; 97: 256-61.
- Zhou X, Ye X, Guo X, Liu D, Xu J, Hu F et al. Safety of SGLT2 inhibitors: A pharmacovigilance study from 2013 to 2021 based on FAERS. Front Pharmacol. 2021; 12: 766125.
- Hu Y, Bai Z, Tang Y, Liu R, Zhao B, Gong J, et al. Fournier gangrene associated with sodium-glucose Cotransporter-2 inhibitors: A pharmacovigilance study with data from the U.S. FDA Adverse Event Reporting System. J Diabetes Res. 2020; 2020: 3695101.
- Yang H, Choi E, Park E, Na E, Chung SY, Kim B, et al. Risk of genital and urinary tract infections associated with SGLT-2 inhibitors as an add-on therapy to metformin in patients with type 2 diabetes mellitus: A retrospective cohort study in Korea. Pharmacol Res Perspect. 2022; 10: e00910.
- Dave CV, Schneeweiss S, Patorno E. Comparative risk of genital infections associated with sodium-glucose co-transporter-2 inhibitors. Diabetes Obes Metab. 2019; 21: 434-8.
- Gadzhanova S, Pratt N, Roughead E. Use of SGLT2 inhibitors for diabetes and risk of infection: analysis using general practice records from the NPS MedicineWise MedicineInsight program. Diabetes Res Clin Pract. 2017; 130: 180-5.
- Liu J, Li L, Li S, Jia P, Deng K, Chen W, et al. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci Rep. 2017; 7: 2824.
- Kusunoki M, Tsutsumi K, Wakazono N, Hisano F, Miyata T. Influence of luseogliflozin on vaginal bacterial and fungal populations in Japanese patients with Type 2 diabetes. J Clin Med Res. 2021; 13: 309-16.
- Rådholm K, Wu JH, Wong MG, Foote C, Fulcher G, Mahaffey KW, et al. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular disease, death and safety outcomes in type 2 diabetes - A systematic review. Diabetes Res Clin Pract. 2018; 140: 118-28.