Review Article
Austin J Surg. 2023; 10(4): 1311.
The Implications of Sarcopenia in the Treatment and Prognosis of Pancreatic Cancer
Elroy Patrick Weledji¹*; Luca Gianotti²; Massimo Oldani²; Fabio Uggeri²
1Department of Surgery, Faculty of Health Sciences, University of Buea, Cameroon, W/Africa
2Department of Surgery, San Gerardo Hospital, Monza, School of Medicine and Surgery, University of Milano-Bicococca, Italy
*Corresponding author: Elroy Patrick Weledji Livanda Kongo Hill, PO Box 126 Limbe, S.W. Region, Cameroon, W/Africa. Tel: 237 699922144 Email: elroypat@yahoo.co.uk
Received: August 01, 2023 Accepted: September 20, 2023 Published: September 27, 2023
Abstract
Sarcopenia is the subclinical loss of skeletal muscle and strength and has been extensively studied in both cancer and surgical patients. Patients with sarcopenia are particularly vulnerable to major physiological stressors including surgery and surgical complications. Sarcopenia has thus gained significant recognition as an important prognostic factor for both complications and survival in cancer patients. The aim of this review was to evaluate the current literature on the effect of sarcopenia on the treatment and prognosis of pancreatic cancer. The prevalence of sarcopenia in pancreatic cancer patients range between 20% to 65% due to the heterogeneous groups of patients, difference in disease stage, and the different methods of measuring sarcopenia. Sarcopenia would be more accurately assessed by utilizing both imaging and clinical data, such as frailty. Although malnutrition could be responsible for the attenuated healing process of pancreatic anastomosis the relationship between sarcopenia and outcome following pancreaticoduodenectomy is debated. Most studies showed a higher risk of Postoperative Pancreatic Fistula (POPF) formation in patients with concurrent sarcopenia and high fat mass (sarcopenic obesity). Sarcopenia seems generally to be associated with lower survival. The assessment of sarcopenia can therefore lead to changes in management strategy, patient selection, and improved informed consent prior to surgical resection of pancreatic cancer. An improved prediction of clinically relevant pancreatic fistula formation after pancreatic surgery using preoperative Computed Tomography (CT) scan, including a fistula risk score using sarcopenic obesity and subcutaneous fat area will be useful. Although treatment for sarcopenia still remains an area of research a protocol to improve nutrition and fitness preoperatively may improve sarcopenia and surgical outcome.
Keywords: Sarcopenia; Sarcopenic obesity; Assessment; Pancreatic cancer; Surgery; Chemotherapy; Outcome
Introduction
Pancreatic cancer is one the most aggressive malignancies with rising incidence. It is the fourth most common cause of cancer death in the Western world because diagnosis is often only established in the advanced stages, and thus the low treatment success rate [1]. Its poor prognosis is manifested in an overall median survival of 4.4 months, and a 5-year survival of 9.7%. In the past 20 years, there is only a modest increase in long-term survival with a median survival of 12 months, and a 5-year survival rate of 15-26% after potentially curative resection [1]. Loss of lean tissue mass (sarcopenia) attributed to malignancy is a well-established complication and has been the focus of a great deal of clinical investigation [2]. Malignancy can result in a hypercatabolic state caused by tumour metabolism, systemic inflammation, and other tumour mediated effects [3]. This derangement in an individual's homeostasis combined with other cancer-mediated effects such as anorexia, fatigue, decreased functional status, and immobility leads to a depletion of skeletal muscle and the development of sarcopenia. Sarcopenia is a syndrome first introduced by Rosenberg in 1989, characterized by progressive and generalized loss of skeletal muscle mass and strength [4]. It is commonly accepted as an age - related process and, in that setting is an important predictor of surgical outcome and discharge destinations [2-6]. There is increasing evidence that the elderly and frail are not the only populations, which suffer from sarcopenia. With an increase in fatty tissue mass: lean tissue mass ratio, patients may also experience sarcopenic obesity. This population is vulnerable to both the adverse health consequences of excess adipose tissue as well as to the complications associated with a decrease in muscle mass [7-9]. Perhaps most striking is the cohort of patients suffering from a malignancy and cancer-related cachexia. The common manifestation of tumour cachexia with an incidence of 60-80%, is a complex syndrome that combines malnutrition with weight loss, decrease in muscle tissue (sarcopenia), anorexia, early satiety, weakness, anaemia and oedema [10]. The impact of sarcopenia in cancer patients has been studied across a broad range of malignancies [8-12], and it has been shown to predict drug toxicity, time to tumour progression, and mortality in patients treated with chemotherapeutic agents [10]. Muscle loss is also exacerbated by the administration of cytotoxic chemotherapy, and an independent prognostic indicator in cancer patients undergoing palliative therapy [11]. While the stepwise progression towards sarcopenia is not yet clearly defined, there is no question of the deleterious effects that it has on clinical outcomes in cancer populations [10,12]. The decision to undergo any surgical intervention is based on weighing the clinical benefits versus potential complications. Patients with sarcopenia are particularly vulnerable to major physiological stressors including surgery and surgical complications [12]. Englesbe et al demonstrated that core muscle size is independently predictive of mortality and complications following major elective general or vascular surgery [13]. Sarcopenia has also been shown to correlate with mortality after liver transplantation, length of stay after colon resection, and surgical site infections following midline laparotomies and colon resections [14,15].
Long-term survival is worse in sarcopenic patients undergoing pancreatic cancer surgery, as shown by meta-analysis [16,17]. As a result the approach towards oncological therapy may be forced towards the use of suboptimal and inadequate treatment. Several factors are considered when evaluating a sarcopenic patient's preoperatively, and include medical co-morbidities and nutritional status [18]. Concomitant with these objective data, is a more subjective ‘eyeball test’ to evaluate for the patient's expected physiologic reserve [18,19]. This will provide the surgeon a more impartial tool for assessing the ability to tolerate surgery. Sarcopenia is a component of body habitus that can be quantified preoperatively and altered over time. The assessment of sarcopenia can lead to changes in management strategy, patient selection, and improved informed consent prior to surgical resection of malignancy. The aim of this review was to discuss the current literature on the association between sarcopenia and surgical outcome following resection of pancreatic cancer.
Discussion
Pancreatic surgery is technically complex and associated with significant postoperative morbidity, mortality, and prolonged hospitalization. Although, in recent decades, survival after pancreatic surgery has improved due to recent advancements in perioperative management and operative technique, post-operative complications occurs in up to 40-50% of patients [20]. Sarcopenia seems to be associated with poorer survival, higher postoperative morbidity, and mortality in patients undergoing pancreatic surgery. The prevalence of sarcopenia in pancreatic cancer patients range from 20% to 65% due to the heterogeneous groups of patients, difference in disease stage, and the different methods of measuring sarcopenia [21-23]. Pancreaticoduodenectomy is the gold standard in the treatment of pancreatic, periampullary, and distal bile duct malignancies. POPF is one of the most common and relevant complications following this procedure. Many possible risk factors have been identified, such as male gender, higher body mass index, prior history of cholangitis, cardiovascular disease, benign rather than malignant indication predisposes narrow pancreatic duct, extrapancreatic tumour location (distal cholangiocarcinoma, ampullary, duodenal) predisposes soft pancreas, blood loss, soft parenchymal texture, narrow pancreatic duct width (<3mm), absence of intraoperative blood transfusion, and higher fluid amylase on postoperative day 1 [24]. The evaluation of the nutritional status of patients undergoing pancreatic surgery has been receiving increasing attention, especially in recent years and according to a position paper of the International Study Group on Pancreatic Surgery (ISGPS), the measurement of nutritional status should be part of the routine preoperative assessment, as malnutrition is a risk factor of surgery-related complications. The group also suggests considering, in addition to the patient’s weight loss and Body Mass Index (BMI), the measurement of sarcopenia and sarcopenic obesity [23,25]. It can be assessed by the routine preoperative staging CT but its role in surgical outcome in particular the occurrence of POPF is still unclear and debatable [26,27]. Predicting POPF using a combination of objective preoperative CT measurements including body composition parameters would still be very useful [26,27].
Method of Quantifying Sarcopenia
Sarcopenia is found in up to 65% of pancreatic cancer patients [28], but there is no standardized methodology for both the assessment and classification of sarcopenia in the clinical setting. The current framework for quantification involves imaging of skeletal muscle and the determination of cut-off values based on individual study populations. There is available evidence on the role of CT scans in both the identification of sarcopenia in patients with abdominal malignancies as well as the predictive value of body composition analysis in clinical outcomes. CT scans can identify reduced muscle mass and predict negative cancer outcomes in patients with abdominal malignancies [26]. The Skeletal Muscle Index (SMI) at the third lumbar vertebra level on preoperative CT was the most common way of assessing sarcopenia, although the cut-offs varied among different studies. In the studies evaluated, imaging modalities used included CT scan, Magnetic Resonance Imaging (MRI), dual energy x-ray absorptiometry, and bioelectrical impedance assay [7] but the majority of studies used CT scans [27]. This can be attributed to the fact that preoperative CT scans are the standard of care for patients undergoing resection of a malignancy. Most studies employed a semi-automated method for taking measurements from the scans; the intended musculature was manually outlined with a preset Hounsfield Unit density threshold. This technique allows for more precise calculation of the muscle area while excluding fat and vasculature that fall outside the preset Hounsfield Unit range [29]. The Hounsfield Unit parameters set by most studies was within -30 to 150 HU [30]. There are several different musculature measurements that are used to quantify sarcopenia. In general, measurements are taken at a particular level of the lumbar spine (primarily L3), or the value is obtained by averaging measurements from two consecutive lumbar vertebral levels (e.g., L4 and L5; Figure 1, Panels A–C). A majority of the studies reviewed obtained the cross sectional area of the abdominal skeletal musculature (including bilateral psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal oblique, and rectus abdominis) or the cross sectional area of the psoas muscles. A few studies defined sarcopenia based on both psoas muscle area and psoas muscle density, expressed in Hounsfield Units. Psoas muscle density is a proxy for muscle quality as it accounts for fatty infiltration of muscle tissue. This is also known as the Hounsfield Unit Average Calculation, or HUAC. Other measurements included the appendicular skeletal muscle mass and the multifidus muscle with subcutaneous fat [31]. An example of how sarcopenia is quantified by CT imaging is illustrated from a study published by Joglekar and co-workers [32]. In this study, sarcopenia was defined as meeting the lower 25th percentile for gender-specific Total Psoas Index (TPI) and HUAC (Figure 1). In Figure 1, Panel A demonstrates a patient with a normal TPA as seen by the substantial muscle mass. The patient shown in Panel B is illustrative of someone with very small TPA and therefore a low TPI (sarcopenia). The patient shown in Panel C has a substantial muscle mass as shown by visual estimation, but the quality of the muscle is low based on the low HUAC and met criteria for sarcopenia [32]. In many of the studies reviewed, sarcopenia was largely defined as a dichotomous variable by establishing cut-off points for the muscle index used. Cut-off values were commonly determined by lowest gender specific quartile optimum stratification to obtain gender specific cut-offs or two standard deviations below the gender specific mean. Of note, numerous studies obtained their cut-off values by using the optimum stratification model outlined by Prado and co-workers [8,9]. Gender specific cutoffs were used due to the baseline variability in body habitus between males and females. Despite the variability in the specifics of the method for quantifying sarcopenia, the studies included in this review all used an imaging modality to obtain measurements of skeletal muscle mass or density and defined cut-off values based on the skeletal muscle index calculated. In 2019, a revised European concensus on the definition and diagnosis of sarcopenia identifies probable sarcopenia by low muscle strength (criterion1), low muscle quantity or quality (criterion 2) and low physical performance (criterion 3). If criteria 1, 2, and 3 are all met, sarcopenia is considered severe. Lumbar third vertebra imaging by CT is considered among the techniques that can be used to detect low muscle mass [2].

Figure 1: Quantification of sarcopenia on contrast enhanced computed tomography in patients with pancreatic adenocarcinoma being considered for pancreatectomy. (TPA, Total Psoas Area, HUAC, Hounsfield Unit Average Calculation, HU, Hounsfield Units) (With permission [31].
Type of Malignancy
Many studies have demonstrated the significant prognostic role of sarcopenia for both cancer-related survival and complications following oncologic procedures [33]. The broad range of percentage of sarcopenia across the studies (11.1% to 68.8%) may be attributed to the lack of a standardized definition of sarcopenia, as well as innate differences in the patient populations evaluated. Given the unique qualities of each solid-type tumour, and the type of procedures sarcopenia will not have the same prognostic value in all types of malignancy [4-8]. Pancreatic cancer has a major impact on the patient’s nutritional status by virtue of their inherent digestive functions. Other factors include disease stage (tumour- related), treatment used (treatment-related) and performance status (patient-related). Patients requiring surgical intervention will further impose metabolic and cardiopulmonary demands that compounds pre-existing nutritional disorders [8,22,33-35]. Malnutrition has to be severe before healing is affected because the wound has high priority when competing with unwounded tissue for body resources [36]. In pancreatic cancer, malnutrition occurs in 60-80% of patients but on its own it should be severe to cause the decrease in muscle mass and functional capacity of sarcopenia that may have an effect on surgical outcome [22]. In addition the loss of stroma in the sarcopenic pancreas (Figure 2) will not render strength in a pancreatic anastomosis. Sarcopenia or a history of rapid onset weight loss or weight loss >20% of original weight is evidence of advanced disease and not simply due to insufficient nutrient intake or nutrient losses. It is associated with poor prognosis i.e. lower survival, worse response to chemotherapy and radiotherapy with increased risk of toxicity, increased risk of post operative complications, delayed wound healing, nosocomial infections and decreased Quality of Life (QoL) [33,36]. In addition, nutrient supplements involved in the healing process may only be effective when these nutrient factors are deficient [22,33,37,38].
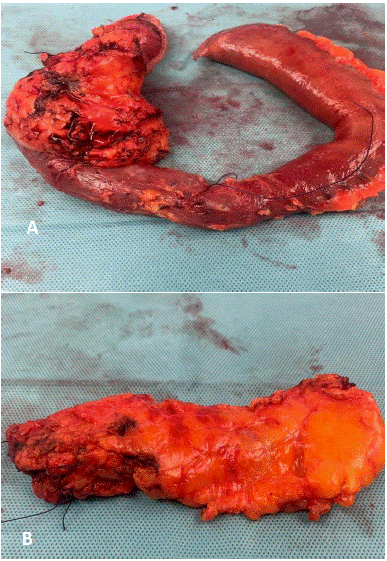
Figure 2: Total Pancreatectomy specimen of sarcopenic patient (minimal stroma and greater fat content): (a) pancreaticoduodenectomy specimen (b) body and tail specimen.
Major Outcome Evaluated in Pancreatic Cancer Patients with and without Sarcopenia
Peng 2012 and Okumura 2015 demonstrated on 557 and 230 pancreatic adenocarcinoma patients respectively that sarcopenia was a prognostic factor for survival following pancreatectomy [39,40]. Joglekar 2015 demonstrated on 118 pancreatic adenocarcinoma patients that sarcopenia was a significant predictor of complications following pancreatectomy [32]. Grading of complications is a critical aspect of any study evaluating outcome across patient groups in surgical oncology patients [34,41]. The most mature and established complications reporting system available is that for pancreatectomy, where there is a specific grading system that has been published and validated across institutions [42,43]. When evaluating pancreatectomy for adenocarcinoma, Joglekar and co-workers [32] utilized two methods for quantification of sarcopenia. The complications were graded according to Common Toxicity for Adverse Events or the International Study Group for Pancreatic Surgery when applicable. The TPI only predicted length of hospital stay on multivariate analysis. However, the HUAC, a measure of muscle quality, was also an independent predictor of length of stay and ICU admission, Clavien-Dindo grade 3 complications, overall complications, delayed gastric emptying, and infectious, gastrointestinal, and cardiopulmonary complications. Overall survival was not found to be different based on the TPI or HUAC in this study. The authors concluded that not only muscle mass, but muscle quality is an important variable in assessment of sarcopenia that should be considered when evaluating patients for pancreatectomy. The prognostic value of sarcopenia on postoperative complications and survival is clinically relevant as it can be objectively and reliably measured and is a potentially modifiable risk factor. While a standard first line therapy for remediating sarcopenia has not yet been identified, several studies have suggested potential interventions. Commonly proposed strategies include a combination of high-protein nutritional support, early physical therapy, and alternative muscle stimulation for the non-ambulatory population [3].
Impact of Sarcopenia on Postoperative Complications in Pancreatic Cancer
Postoperative Pancreatic Fistula (POPF) is one of the most critical complications after pancreatic surgery [17]. To evaluate the susceptibility to POPF, the Fistula Risk Score (FRS) has been designed, taking four risk factors into consideration: (a) the texture of the pancreas, (b) disease pathology, (c) pancreatic duct size, (d) intraoperative blood loss[44]. In addition, several surgical strategies such as anastomotic stents for pancreatic duct width (<3mm), tissue sealants and autologous tissue patches have been introduced to decrease the incidence of clinically relevant POPF [45]. In a meta-analysis, Zhao et al [46] concluded that external drainage of the pancreatic juice was not superior to internal drainage in reducing the incidence of POPF and, external drainage did not decrease the incidence of CR-POPF for soft pancreatic gland texture [47]. Although malnutrition could be responsible for the attenuated healing process of pancreatic anastomosis the relationship between sarcopenia and outcome following pancreaticoduo denectomy is debated. Only 6 of the 21 studies observed a statistically significant effect of sarcopenia, but the data showed an unclear picture on its role in POPF formation. Latorre Fragua et al’s systematic review in 2022 [20] demonstrated that sarcopenia was not associated with an increased incidence of the specific postoperative complications, such as pancreatic fistula, delayed gastric emptying, sepsis, postoperative haemorrhage or mortality. However, routine preoperative staging CT could improve preoperative risk stratification in patients undergoing pancreatic surgery [20]. Most studies showed a higher risk of POPF in patients with sarcopenic obesity [48-51]. According to Nishida et al [52] preoperative sarcopenia strongly influences the risk of postoperative pancreatic fistula formation after pancreaticoduodenectomy, and for Linder et al [53], sarcopenia contributed to the occurence of POPF, while Amrani et al [54], Sui et al [55], Box et al [56], and Tsukagoshi et al [57] reported that sarcopenia was a protective factor for POPF. Centoze et al [58] showed a significant difference only for grade C POPF. It is important to note that the occurrence of POPF after Pancreaticoduo Denectomy (PD) has different causative factors to Distal Pancreatectomy (DP). 17 of the 21 studies were conducted on patients undergoing PD but only 5 studies reported a significant difference in POPF between the two groups [52,53,55-57]. The only study that included patients undergoing distal pancreatectomy did not find a significant association between sarcopenia and POPF formation [59]. A trend of a lower POF rate in sarcopenic patients may be explained by the perioperative nutritional supplementation in sarcopenic patients but more studies are needed to classify these findings [23]. Thus in order to adequately assess the role of sarcopenia researchers should also evaluate the data on the state of nutrition of patients, parenteral and jejunostomy enteral nutrition. Although sarcopenia is known to be associated with higher mortality and functional disability [52], it is becoming increasingly evident that concurrent sarcopenia and high fat mass (sarcopenic obesity) is the worst case scenario [7,11,22,48-51]. To avoid the risk of POPF, three studies -Joglekar et al 2015 [31], Okumura et al 2015 [40], Amrani et al 2018 [54] demonstrated total pancreatectomy in sarcopenia. In addition to the report that skeletal muscle quality is associated with worse survival after pancreaticoduodenectomy for periampullary, non-pancreatic cancers [60] performing a spleen-preserving total pancreatectomy for distal cholangiocarcinoma in a sarcopenic insulin-dependent diabetic patient was demonstrated by the authors (unpublished) to be safe and effective (Figure 2).
Impact of Sarcopenia on Postoperative Survival in Pancreatic Cancer
Several studies evaluated the impact of sarcopenia on postoperative cancer-specific and overall survival and was shown to be independently associated with survival in several of the studies reviewed [40,41-69]. The study by Peng and co-workers observed a 63% increased risk of death at 3 years in sarcopenic patients with pancreatic ductal adenocarcinoma [40]. Consistent with these findings, Sabel and co-workers found that with every 10 HU decrease in psoas muscle density there was a 28% decrease in disease-free survival [70]. The findings in these studies controlled for complications and other significant prognostic factors using multivariate analysis demonstrated that sarcopenia is a significant prognostic factor for survival. No significant difference in postoperative mortality was among the studies that presented 60 days mortality. When only patients with PD were analyzed, patients with sarcopenia showed poorer overall survival than those without sarcopenia. Meanwhile in patients with distal pancreatectomy, there was no difference in survival rates between the two groups [71-75] (Table 2).
Is It Possible to Minimize the Impact of Sarcopenia on Patients Undergoing Pancreatic Surgery?
Generally, exercise and nutritional management are crucial for the prevention and treatment of sarcopenia. Nutritional therapy for sarcopenia that includes 20g of whey protein and 800 IU of vitamin D twice a day improves muscle strength and physical function but may take up to 6 months to be most effective [76]. Appropriate nutritional management and exercise programs through rehabilitation nutrition should therefore be started very early after admission and adjusted to the level of the pancreatic cancer disease status [77,78]. The evaluation of the TPA score (Total Psoas muscle) the most sensitive marker for detecting pre-sarcopenia preoperatively may identify priority patients who might benefit from pre-habilitation programs [30]. Several studies have shown a worse survival outcome and an increase in postoperative complications based on body composition measurement in patients with pancreatic adenocarcinoma, regardless of stage or treatment modality [22,23,27,28,32,40,51-54,56,57,61-64].
This raises the question of how to proceed with patients who have sarcopenia in pancreatic cancer. The use of an intervention program would be an ideal option for patients with severe sarcopenia. An example is a patient with resectable pancreatic head adenocarcinoma who has been deemed an operative candidate and yet has severe sarcopenia. Because sarcopenia is mostly associated with advanced disease the initiation of systemic chemotherapy along with a protocol to improve nutrition and fitness preoperatively, followed by restaging and reassessment of sarcopenia seem reasonable [8,23,79,80]. Although it is possible that sarcopenic patients may not tolerate chemotherapy as well as non-sarcopenic patients, more centers have published on improved outcome with neoadjuvant chemotherapy for pancreatic adenocarcinoma in sarcopenic patients [28,81]. Serum albumin and protein levels are always considered preoperatively and surgery can be delayed safely on patients with albuminaemia <2.8g/dl and proteinaemia <5.5g/dl for provision of high protein nutritional supplements. Medications to reduce the risk of POPF formation such as somatostatin analogues could be used for prophylaxis in selected patients [23,42].
The Future
Whether sarcopenia is a determinant or merely a predictor associated with survival remains unknown, and future studies may help clarify the significance. For future studies, it would be valuable to have a universal method for quantifying sarcopenia and determining standardized cutoff values that can be reliably reproduced across institutions.
A majority of studies have defined sarcopenia as a dichotomous variable, but it can also be utilized as a continuous variable to optimize the cut-offs for each individual study. The use of a standardized gender specific cut-off value would potentially reduce bias across studies but may not be practical due to the heterogeneity of imaging modalities, patients, and cancer subtypes. In addition to imaging measurements, the European Consensus Definition enlists the criteria for the diagnosis of sarcopenia as the presence of low muscle mass and one of the following- low muscle strength or low physical performance [2]. This is a critical aspect of the evaluation of patients that must be considered.
The improvement of prediction of clinically relevant pancreatic fistula after pancreatic surgery using preoperative CT scan [82] and, the application of the new fistula risk score using sarcopenic obesity and subcutaneous fat area will be very useful [83]. Future prospective studies may more accurately assess sarcopenia by utilizing both imaging and clinical data, such as frailty [18]. Therefore, clinical data combined with imaging criteria for sarcopenia should guide patient selection for treatment. There is also a need for the development of a therapeutic strategy to improve the extent of a patient's sarcopenia. If a preoperative protocol were developed, prospective studies analyzing patients in treatment versus control arms might determine whether treating sarcopenia alters a patient's postoperative clinical outcome. Pharmacological therapies for sarcopenia including inhibitors of myostatin, testosterone, selected androgen receptor modulators, ghrelin agonists, and Angiotensin-Converting Enzyme (ACE) inhibitors have been evaluated, but preliminary trials have found they are less effective than postulated [20].
Conclusions
There is increasing evidence that sarcopenia should be considered in the preoperative risk assessment and treatment decision making in patients undergoing pancreatic surgery. Clinical information on sarcopenia may help improve the assessment of a patient's preoperative status, selection for surgical resection, and the determination of timing of multimodality therapy. Although treatment for sarcopenia still remains an area of research, neoadjuvant chemotherapy could be combined with an intensive program of nutrition and exercise, followed by restaging and reassessment of sarcopenia in pancreatic cancer.
Author Statements
Ethical Approval
No ethical approval was required as it is a simple review article.
Author Contribution
EPW substantially contributed to the conception, design and literature search; LG was the main surgeon and reviewed the surgical aspect of the debate, MO was the assisting surgeon and contributed to literature search, FU was the assisting surgeon and contributed to literature search.
Conflict of Interest
The authors have no conflict of interest.
Guarantor
Prof Halle Ekane, Dean, Faculty of Health Sciences, University of Buea, Cameroon.
Research Registration UIN
This was not required in this review article.
References
- Weledji EP, Enow Orock G, Mokake M, Sinju M. How grim is pancreatic cancer? Oncol Rev. 2016; 10: 294.
- Cruz-Jentoft AJ, Bahat G, Bauer JM, et al. Writing Group for the European Working group on Sarcopenia in older people 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019; 48: 16-31.
- Hanna JS. Sarcopenia and critical illness: A deadly combination in the elderly. JPEN J Parenter Enter Nutr. 2015; 39: 273-81.
- Rosenberg IH. Summary comments. Am J Clin Nutr. 1989; 50: 1231-3.
- Fairchild B, Webb TP, Xiang Q, Tarima S, Brasel KJ. Sarcopenia and frailty in elderly trauma patients. World J Surg. 2015; 39: 373-9.
- Du Y, Karvellas CJ, Baracos V, Williams DC, Khadaroo RG, Acute Care and Emergency Surgery (ACES) Group. Sarcopenia is a predictor of outcomes in very elderly patients undergoing emergency surgery. Surgery. 2014; 156: 521-7.
- Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: A critical appraisal of the current evidence. Clin Nutr. 2012; 31: 583-601.
- Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008; 9: 629-35.
- Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009; 15: 6973-9.
- Anjanappa M, Corden M, Green A, Roberts D, Hoskin P, McWilliam A, et al. Sarcopenia in cancer: risking more than muscle loss. Tech Innov Patient Support Radiat Oncol. 2020; 16: 50-7.
- Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009; 15: 2920-6.
- Friedman J, Lussiez A, Sullivan J, Wang S, Englesbe M. Implications of sarcopenia in major surgery. Nutr Clin Pract. 2015; 30: 175-9.
- Englesbe MJ, Lee JS, He K, Fan L, Schaubel DE, Sheetz KH, et al. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg. 2012; 256: 255-61.
- Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010; 211: 271-8.
- Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012; 107: 931-6.
- Ratnayake CBb, Loveday BPT, Shrikhande SV, et al. Impact of preoperative sarcopenia on postoperative outcomes following pancreatic resection: A systematic review and meta-analysis. Panceatology 2020; 20: 1558-1565.
- Perra T, Sotgiu G, Porcu A. Sarcopenia and risk of pancreatic fistula after pancreatic surgery; A systematic review. J Clin Med. 2022; 11: 4144.
- Dale W, Hemmerich J, Kamm A, Posner MC, Matthews JB, Rothman R, et al. Geriatric assessment improves prediction of surgical outcomes in older adults undergoing pancreaticoduodenectomy: A prospective cohort study. Ann Surg. 2014; 259: 960-5.
- Englesbe MJ, Terjimanian MN, Lee JS, Sheetz KH, Harbaugh CM, Hussain A, et al. Morphometric age and surgical risk. J Am Coll Surg. 2013; 216: 976-85.
- Latorre Fragua RA, Manuel Vázquez A, Ramiro Pérez C, de la Plaza Llamas R, Ramia ángel JM. Influence of sarcopenia in major pancreatic surgery. A systematic review of the literature. Gastroenterol Hepatol. 2020; 43: 142-54.
- Bundred J, Kamarajah SK, Roberts KJ. Body composition assessment and sarcopenia in patients with pancreatic cancer: a systematic review and meta-analysis. HPB (Oxford). 2019; 21: 1603-12.
- Gianotti L, Besselink MG, Sandini M, Hackert T, Conlon K, Gerritsen A, et al. Nutritional support and therapy in pancreatic surgery: A position paper of the International study Group on Pancreatic Surgery (ISGPS). Surgery. 2018; 164: 1035-48.
- Kamarajah SK, Bundred JR, Lin A, Halle-Smith J, Pande R, Sutcliffe R, et al. PARANOIA Study group systematic review and meta-analysis of factors associated with post-operative pancreatic fistula following pancreatoduodenotomy. ANZ J Surg. 2021; 91: 810-21.
- Perra T, Porcu A. State of the art in pancreatic surgery; some unanswered questions. J Clin Med. 2022; 11: 2821.
- Gibson DJ, Burden ST, Strauss BJ, Todd C, Lal S. The role of computed tomography in evaluating body composition and the influence of reduced muscle mass on clinical outcome in abdominal malignancy: A systematic review. Eur J Clin Nutr. 2015; 69: 1079-86.
- Aslani A, Gill AJ, Roach PJ, Allen BJ, Smith RC. Preoperative body composition is influenced by the stage of operable pancreatic adenocarcinoma but does not predict survival after Whipple’s procedure. HPB (Oxford). 2010; 12: 325-33.
- Chan MY, Chok KSH. Sarcopenia in pancreatic cancer- effects on surgical outcomes and chemotherapy. World J Gastrointest Oncol. 2019; 11: 527-37.
- Namm JP, Thakrar KH, Wang CH, Stocker SJ, Sur MD, Berlin J, et al. A semi-automated assessment of sarcopenia using psoas area and density predicts outcomes after pancreaticoduodenectomy for pancreatic malignancy. J Gastrointest Oncol. 2017; 8: 936-44.
- Linder N, Schaudinn A, Langenhan K, Krenzien F, Hau HM, Benzing C, et al. Power of computed-tomography-defined sarcopenia for prediction of morbidity after pancreaticoduodenectomy. BMC Med Imaging. 2019; 19: 32.
- Bougard M, Barbieux J, Goulin J, Parot-Schinkel E, Vielle B, Lermite E. The TPA score (total psoas muscle area) is the best marker for preoperative measurement of pre-sarcopenia in pancreatic surgery. J Visc Surg. 2023; 160: 4-11.
- Joglekar S, Asghar A, Mott SL, Johnson BE, Button AM, Clark E, et al. Sarcopenia is an independent predictor of complications following pancreatectomy for adeno-carcinoma. J Surg Oncol. 2015; 111: 771-5.
- Vitali GC, Ronot M, Assalino M, Toso C, Morel P, Berney T, et al. Sarcopenia is a predictor of pancreatic fistula occurrence after duodenopancreatectomyHPB. 2016; 18: E385.
- Mariette C, Alves A, Benoist S, Bretagnol F, Mabrut JY, Slim K, et al. Perioperative care in digestive surgery. J Chir (Paris). 2005; 142: 14-28.
- Weledji EP, Ngowe MM. The impact of nutritional status on the multimodal treatment of oesophageal and gastric cancer. Austin Surg Oncol. 2017; 2: 1008.
- Weledji EP, Verla V. Failure to rescue patients from early critical complications of oesophagogastric cancer surgery. Ann Med Surg (Lond). 2016; 7: 34-41.
- Weledji EP. Perspectives on wound healing. Austin J Surg. 2017; 4: 1104.
- Friedman J, Lussiez A, Sullivan J, Wang S, Englesbe M. Implications of sarcopenia in major surgery. Nutr Clin Pract. 2015; 30: 175-9.
- Thompson C, Fuhrman MP. Nutrients and wound healing; still searching for the magic bullet. Nutr Clin Pract. 2005; 20: 337-47.
- Peng P, Hyder O, Firoozmand A, Kneuertz P, Schulick RD, Huang D, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012; 16: 1478-86.
- Okumura S, Kaido T, Hamaguchi Y, Fujimoto Y, Masui T, Mizumoto M, et al. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery. 2015; 157: 1088-98.
- Grobmyer SR, Pieracci FM, Allen PJ, Brennan MF, Jaques DP. Defining morbidity after pancreaticoduodenectomy: use of a prospective complication grading system. J Am Coll Surg. 2007; 204: 356-64.
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005; 138: 8-13.
- Mezhir JJ. Management of complications following pancreatic resection: an evidence-based approach. J Surg Oncol. 2013; 107: 58-66.
- Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013; 216: 1-14.
- Poon RTP, Fan ST, Lo CM, Ng KK, Yuen WK, Yeung C, et al. External drainage of pancreatic duct with a stent to reduce leakage rate of pancreaticojejunistomy after pancreaticoduodenectomy: A prospective randomized trial. Ann Surg. 2007; 246: 425-33.
- Zhao Y, Zhang J, Lan Z, Jiang Q, Zhang S, Chu Y, et al. Are Internal or external duct stents the preferred choice for patients undergoing pancreaticoduodenectomy? A meta-analysis. BioMed Res Int. 2017; 2017: 13672381.
- Gu J, Du J, Xie Z, Zou C, He H, Li H, et al. A retrospective study comparing external and internal without stent pancreatic drainage after pancreatic operation. Surg Pract Sci. 2020; 1: 1000009.
- Ryu Y, Dhin SH, Kim JH, Jeong WK, Park DJ, Kim N, et al. The effects of sarcopenia and sarcopenic obesity after pancreaticoduodenectomy in patients with pancreatic head cancer. HPB. 2020; 22: 1782-1792.
- Pecorelli N, Carrara G, De Cobelli F, Cristel G, Damascelli A, Balzano G, et al. Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br J Surg. 2016; 103: 434-42.
- Jang M, Park HW, Huh J, Lee JH, Jeong YK, Nah YW, et al. Predictive value of sarcopenia and visceral obesity for postoperative pancreatic fistula after pancreaticoduodenectomy analysed on clinically acquired CT and MRI. Eur Radiol. 2019; 29: 2417-25.
- Mintziras I, Miligkos M, Wächter S, Manoharan J, Maurer E, Bartsch DK. Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer:systematic review and meta-analysis. Int J Surg. 2018; 59: 19-26.
- Nishida Y, Kato Y, Kudo M, Aizawa H, Okubo S, Takahashi D, et al. Preoperative sarcopenia strongly influences the risk of postoperative fistula formation after pancreaticoduodenectomy. J Gastrointest Surg. 2016; 20: 1586-94.
- Linder N, Schaudinn A, Langenhan K, Krenzien F, Hau HM, Benzing C, et al. Power of computed-tomography-defined sarcopenia for prediction of morbidity after pancreaticoduodenectomy. BMC Med Imaging. 2019; 19: 32.
- El Amrani M, Vermersch M, Fulbert M, Prodeau M, Lecolle K, Hebbar M, et al. Impact of sarcopenia on outcomes of patients undergoing pancreatectomy: A retrospective analysis of 107 patients. Medicine. 2018; 97: e12076.
- Sui K, Okabayshi T, Iwata J, Morita S, Sumiyoshi T, Iiyama T, et al. Correlation between the skeletal muscle index and surgical outcomes of pancreaticoduodenectomy. Surg Today. 2018; 48: 545-51.
- Box EW, Deng L, Morgan DE, Xie R, Kirklin JK, Wang TN, et al. Preoperative anthropomorphic radiographic measurements can predict postoperative pancreatic fistula formation following pancreatoduodenectomy. Am J Surg. 2021; 222: 133-8.
- Tsukagoshi M, Harimoto N, Araki K, Kubo N, Watanabe A, Igarashi T, et al. Impact of preoperative nutritional support and rehabilitation therapy in patients undergoing pancreaticoduodenectomy. Int J Clin Oncol. 2021; 26: 1698-706.
- Centonze L, Di Sandro S, Lauterio A, De Carlis R, Botta F, Mariani A, et al. The Impact of sarcopenia on postoperative Course following pancreatoduodenectomy: single-Center Experience of 110 Consecutive Cases. Dig Surg. 2020; 37: 312-20.
- Vanbrugghe C, Ronot M, Cauchy F, Hobeika C, Dokmak S, Aussilhou B, et al. Visceral obesity and open passive drainage increase the risk of pancreatic fistula following distal pancreatectomy. J Gastrointest Surg. 2019; 23: 1414-24.
- Van Rijssen LB, van Huijgevoort NC, Coelen RJ, Tol JA, Haverkort EB, Nio CY, et al. Skeletal muscle quality is associated with worse survival after pancreatoduodenectomy for periampullary, nonpancreatic cancer. Ann Surg Oncol. 2017; 24: 272-80.
- Sandini M, Bernasconi DP, Fior D, Molinelli M, Ippolito D, Nespoli L, et al. A high visceral adipose tissue-to-skeletal muscle ratio as a determinant of major complications after pancreatoduodenectomy for cancer. Nutrition. 2016; 32: 1231-7.
- van Dijk DP, Bakens MJ, Coolsen MM, Rensen SS, van Dam RM, Bours MJ, et al. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J Cachexia Sarcopenia Muscle. 2017; 8: 317-26.
- Takagi K, Yoshida R, Yagi T, Umeda Y, Nobuoka D, Kuise T, et al. Radiographic sarcopenia predicts postoperative infectious complications in patients undergoing pancreaticoduodenectomy. BMC Surg. 2017; 17: 64.
- Fukuda Y, Asaoka T, Eguchi H, Sasaki K, Iwagami Y, Yamada D, et al. Clinical impact of preoperative sarcopenia on the postoperative outcomes after pancreas transplantation. World J Surg. 2018; 42: 3364-71.
- Tanaka K, Yamada S, Sonohara F, Takami H, Hayashi M, Kanda M, et al. Pancreatic fat and body composition measurements by computed tomography are associated with pancreatic fistula after pancreatectomy. Ann Surg Oncol. 2021; 28: 530-8.
- Yamane H, Abe T, Amano H, Hanada K, Minami T, Kobayashi T, et al. Visceral adipose tissue and skeletal muscle index distribution predicts severe pancreatic fistula development after pancreaticoduodenectomy. Anticancer Res. 2018; 38: 1061-6.
- Abe T, Amano H, Kobayashi T, Hanada K, Hattori M, Nakahara M, et al. Preoperative anthropomorphic and nutritious status and fistula risk score for predicting clinically relevant postoperative pancreatic fistula after pancreaticoduodenectomy. BMC Gastroenterol. 2020; 20: 264.
- Roh YH, Kang BK, Song SY, Lee CM, Jung YK, Kim M. Preoperative CT anthropometric measurements and pancreatic pathology increase risk for postoperative pancreatic fistula in patients following pancreaticoduodenectomy. PLOS ONE. 2020; 15: e0243515.
- Sabel MS, Lee J, Cai S, Englesbe MJ, Holcombe S, Wang S. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol. 2011; 18: 3579-85.
- Onesti JK, Wright GP, Kenning SE, Tierney MT, Davis AT, Doherty MG, et al. Sarcopenia and survival in patients undergoing pancreatic resection. Pancreatology. 2016; 16: 284-9.
- Amini N, Spolverato G, Gupta R, Margonis GA, Kim Y, Wagner D, et al. Impact total psoas volume on short- and long-term outcomes in patients undergoing curative resection for pancreatic adenocarcinoma: a new tool to assess sarcopenia. J Gastrointest Surg. 2015; 19: 1593-602.
- Ninomiya G, Fujii T, Yamada S, Yabusaki N, Suzuki K, Iwata N, et al. Clinical impact of sarcopenia on prognosis in pancreatic ductal adenocarcinoma: a retrospective cohort study. Int J Surg. 2017; 39: 45-51.
- Stretch C, Aubin JM, Mickiewicz B, Leugner D, Al-Manasra T, Tobola E, et al. Sarcopenia and myosteatosis are accompanied by distinct biological profiles in patients with pancreatic and periampullary adenocarcinomas. PLOS ONE. 2018; 13: e0196235.
- Choi MH, Yoon SB, Lee K, Song M, Lee IS, Lee MA, et al. Preoperative sarcopenia and post-operative accelerated muscle loss negatively impact survival after resection of pancreatic cancer. J Cachexia Sarcopenia Muscle. 2018; 9: 326-34.
- Collins JT, Noble S, Chester J, Davies HE, Evans WD, Lester J, et al. Association of sarcopenia and observed physical performance with attainment of multidisciplinary team planned treatment in non-small cell lung cancer: an observational study protocol. BMC Cancer. 2015; 15: 544.
- Nakahara S, Takasaki M, Abe S, Kakitani C, Nishioka S, Wakabayashi H, et al. Aggressive nutrition therapy in malnutrition and sarcopenia. Nutrition. 2021; 84: 111109.
- Kakehi S, Wakabayashi H, Inuma H, Inose T, Shioya M, Aoyama Y, et al. Rehabilitation nutritition and Exercise therapy for sarcopenia. World J Mens Health. 2022; 40: 1-10.
- Piastra G, Perasso L, Lucarini S, Monacelli F, Bisio A, Ferrando V, et al. Effects of two types of 9-month adapted physical activity program on muscle mass, muscle strength, and balance in moderate sarcopenic older women. BioMed Res Int. 2018; 2018: 5095673.
- Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009; 12: 86-90.
- Sadot E, Doussot A, O’Reilly EM, Lowery MA, Goodman KA, Do RK, et al. FOLFIRINOX induction therapy for stage 3 pancreatic adenocarcinoma. Ann Surg Oncol. 2015; 22: 3512-21.
- Arvold ND, Ryan DP, Niemierko A, Blaszkowsky LS, Kwak EL, Wo JY, et al. Long-term outcomes of neoadjuvant chemotherapy before chemoradiation for locally advanced pancreatic cancer. Cancer. 2012; 118: 3026-35.
- Zhang X, Yu H, Song B. Prediction of clinically relevant pancreatic fistula after pancreatic surgery using preoperative CT scan: A systematic review and meta-analysis. Yue Y. Li M. Pancreatology. 2022; 20: 1558-65.
- Hayashi H, Shimizu A, Kubota K, Notake T, Masuo H, Yoshizawa T, et al. A new fistula risk score using sarcopenic obesity and subcutaneous fat area of predicting postoperative pancreatic fistula after pancreaticoduodenectomy. J Hepato-Bil Pancreat Sci. 2003; 30: 792-801.