
Research Article
J Stem Cells Res, Rev & Rep. 2014;1(1): 1005.
Ifnβ Regulates Human Bone Marrow Derived Mesenchymal Stem Cell Differentiation: Interaction with Canonical Wnt Signaling
Weimin Qiu1*, Li Chen1 and Moustapha Kassem1-3
1Department of Endocrinology, Odense University Hospital, Denmark
2Department of Anatomy, King Saud University, Saudi Arabia
3Department of Health Sciences, University of Copenhagen, Denmark
*Corresponding author: Weimin Qiu, Department of Endocrinology, Molecular Endocrinology Laboratory (KMEB), Odense University Hospital, Winsløws parken 25.1, DK-5000 Odense C, Denmark
Received: Aug 07, 2014; Accepted: Aug 20, 2014; Published: Aug 21, 2014
Abstract
Interferons (IFNs) are multifunctional secreted cytokines involved in antiviral defense, cell growth regulation and immune activation. In addition, IFNs have been reported to play a regulatory role in bone homeostasis and bone resorption. However, the effects of IFNs on osteoblastic cells and bone formation are not clear. In the current study, we demonstrated that IFNβ decreased Human Bone Marrow derived mesenchymal Stem Cell (hBMSC) proliferation and their osteoblast differentiation but enhanced adipocyte differentiation by inhibiting Wnt signaling. Wnt signaling inhibition was mediated by two mechanisms: upregulation of Wnt antagonist DKK via canonical IFN signaling and reduction of stabilized nuclear β-catenin via reduced AKT phosphorylation. Targeting IFNβ signaling in hBMSC is a potential approach to enhance bone formation.
γKeywords: Human bone marrow derived mesenchymal stem cell; IFNβ; Wnt; Bone homeostasis
Introduction
Osteoimmunology is an interdisciplinary research area focusing on interaction of the immune system with bone and initially transpired due to the close proximity of bone and bone marrow organ. An increasing number of immune related cytokines have been reported to play a regulatory role in bone biology [1,2]. Among these molecules are Interferons (IFNs) which are multifunctional secreted cytokines involved in antiviral defense, cell growth regulation and immune activation. IFNs are categorized into two classes: type I IFN are produced in direct response to virus infection and are comprised of IFNα, IFNβ, and type II IFN consists of IFNγ which is produced in response to the recognition of infected cells by activated T lymphocytes and natural killer cells [3]. All type I IFNs share a common type I IFN receptor whereas IFNγ binds to type II IFN receptor [4]. Several studies have demonstrated that IFNs exert direct effects on bone cells. IFNs regulate osteoclast formation and osteoclastic bone resorption [2,5]. IFNγ promotes bone formation and rescues osteoporosis-related bone loss in ovariectomized mice [6,7]. IFNγ has also been reported to inhibit adipogenesis of Human Bone Marrow derived mesenchymal Stem Cells (hBMSC) in vitro and to prevent bone marrow fat infiltration in ovariectomized mice [8]. Mice deficient in IFNα receptor 1 (IFNAR1) (IFNAR1-/-) develop osteoporosis due to enhanced osteoclastic bone resorption [9]. A similar low bone mass phenotype was observed in IFNβ-/- mice (9). IFNβ has been reported to inhibit osteoblast-mediated mineralized matrix formation in vitro - effects that were abolished by 1α-25- dihydroxyvitamin D3 treatment [10, 11].
During the recent years, Wnt signaling has been recognized as the principal signaling pathway regulating osteoblastic cell differentiation and functions as well as bone formation [12]. Wnt signaling determines MSC differentiation fate to osteoblast or adipocyte lineages [13,14]. Patients with inactivating or activating mutations in Wnt coreceptor lipoprotein related receptor protein 5 (LRP5) causing inactivation or activation of Wnt signaling [15] exhibit either osteoporosis (a disease known as osteoporosis-pseudoglioma syndrome) or high bone mass, respectively [16-19]. Finally, in a number of genetic mouse models, inducible increase or decrease in skeletal Wnt signaling resulted in corresponding changes in bone mass [20-23]. The interaction between IFNs and Wnt signaling has been reported in a number of non-skeletal systems. Nava et al. have shown that, in intestinal epithelium cells, IFNγ induced Wnt signaling by activating PI3K/ AKT/β-catenin pathway and increased expression of DKK1 [24]. Also, IFNα inhibited Wnt signaling in pre-neoplastic rat liver by promoting β-catenin binding to FOXO instead of TCF protein [25] and by upregulating β-catenin nuclear export factor RanBP3 [26]. The interaction of IFN and Wnt signaling in bone has not previously been studied.
In the present study, we investigated the IFN effect on hBMSC proliferation and differentiation and its interaction with Wnt signaling. We demonstrated that among different IFNs, IFNβ exhibited the most significant inhibitory effect on hBMSC proliferation, osteoblast differentiation but enhanced adipocyte differentiation. These effects were mediated through inhibition of Wnt signaling by upregulating the expression of Wnt antagonist DKK and reducing nuclear β-catenin.
Material and Methods
Recombinant proteins, antibodies and conditioned medium
Recombinant human IFNα-2a (Genscript), IFNβ and IFNγ (Peprotech) were reconstituted in 0.1% BSA in 100 μg/ml and stored at -80°C for use. Antibodies for total and phospho-AKT (Ser473), total and phospho-β-catenin (Ser552) were purchased from Cell Signaling. α-tubulin antibody and peroxidase-conjugated secondary antibody were purchased from Santa Cruz. Control Conditioned Medium (CCM) and Wnt3a conditioned medium (Wnt3a) were prepared as previously reported [14].
Cell culture and differentiation
The previous, well characterized hBMSC, which was isolated from a healthy male donor and immortalized by human telomerase approved by local ethics committee [27] was used in this study. The culture and differentiation of hBMSC had been described previously (14). Briefly, cells were cultured in Minimum Essential Medium (MEM) plus 10% FBS and 1% Penicillin/Streptomycin (Invitrogen). Osteogenic induction medium contains 10mM β-glycerophosphate, 50μg/ml 2-phoshate ascorbate, 10nM dexamethasone and 10nM vit D3. Adipogenic induction medium contains 10%FBS, 10%horse serum, 100nM dexamethasone, 450μM 1-methyl-3-isobutylxanthine (IBMX) (Sigma-Aldrich), 1 μM Rosiglitazone (BRL49653) (kindly provided by Novo Nordisk, Bagsvaerd Denmark), 3μg/ml human recombinant insulin (Sigma-Aldrich). In this study, 1ng/ml IFNα, β or γ were also used in corresponding hBMSC differentiation experiments.
Cell proliferation assay
Cell proliferation was determined by CellTiter-Blue reagent. Briefly, hBMSC cells were seeded at 3000 cells/cm2 in 96-well plate with normal culture medium. The next day, attached cell numbers were quantitated by CellTiter-Blue reagent (Promega) according to the instruction as day 0. Medium was changed in the remaining wells to normal culture medium with 0.1%BSA as control or with 1ng/ml or 100ng/ml IFNα, β or γ. Cell number was quantitated at day 1, 3, 5, 7 and 9 and the medium was changed every three days.
ALP activity assay and real-time qRT-PCR analysis
Analysis of osteoblastic and adipocytic marker genes have been described previously [14,28]. Briefly, total RNA was isolated by GenElute™ mammalian total RNA miniprep kit (Sigma) and quantitated by Nanodrop as instructed. Up to 1µg total RNA was reverse transcribed by using iScript cDNA synthesis kit (Bio-Rad) and gene expression was analyzed by using fast SYBR® green master mix (Applied Biosystem) on Step One PlusTM system (Applied Biosystem) according to the manufacturer’s protocol. The primers used in this study were listed in supplemental Table 1.
siRNA knock down
siRNA targeting human type I IFN receptors (IFNAR1 and IFNAR2) and AKT1 (siRNA ID# s7183, s7184, s659 respectively) as well as non-targeting siRNA were purchased from Ambion. 33nM siRNA was reverse transfected by LipofectaminTM 2000 (Invitrogen) according to the instruction.
Luciferase reporter cell lines and luciferase assay
To establish type I IFN and canonical Wnt luciferase reporter cell lines, hBMSC were first infected with lentivirus expressing Renilla Luciferase (GenTarget Inc.) at multiplicities of infection (MOI) of 25 in the presence of 6µg/ml polybrene, and then selected by 300ug/ml G418 (Invitrogen) for one week. Then cells were infected with Cignal Lenti ISRE Luc Reporter or Cignal Lenti TCF/LEF Luc Reporter (CLS-008L or CLS-018L, SABiosciences) respectively and selected by 0.8ug/ml puromycin for one week and then named as hBMSC-ISRE or hBMSC-TCF respectively.
For luciferase assay, reporter cells were seeded in 96-well plate at the density of 2x104 cells/cm2. The next day hBMSC-TCF cells were treated with 5% to 50% Wnt3a conditioned medium, or 20% Wnt3a conditioned medium with 1 or 100ng/ml IFNα, β or γ for 24 hours. To determine IFNAR1 and 2 knocking down efficiency, hBMSC-ISRE reporter cells were transfected by siRNA and treated with 1ng/ml IFNβ for 24 hours. To examine the effect of IFNAR1 and 2 knocking down on Wnt signaling, hBMSC-TCF cells were transfected by siRNA and treated with 20% Wnt3a conditioned medium plus 1ng/ml IFNβ for 24 hours. Luciferase activity was determined by dual luciferase assay (Promega) as described.
Expression analysis of Wnt components
For expression of Wnt components, hBMSC was cultured in 20% Wnt3a conditioned as control or 20% Wnt3a conditioned medium with 1ng/ml IFNβ for three days. Gene expression was analyzed by real-time qRT-PCR [14,28].
Western blot and immunofluorescence staining
hBMSC was treated with either 0.1% BSA as control or 1ng/ ml IFNα, β or γ for 24 hours. Western blot analysis was performed as described [14] and band intensity was quantitated by Image J software and normalized against tubulin. For immunofluorescence staining of nuclear β-catenin, hBMSC was treated with 20% Wnt3a conditioned medium without or with 1ng/ml IFNβ for 2 hours and fixed in PBS buffered formaldehyde (pH 7.0) for 10 minutes followed by incubation with TBS buffered Triton X-100 (pH 7.4) for 10 minutes. After blocking with PBS containing 10% donkey serum for 30 minutes, cells were incubated with anti-β-catenin antibody (1:100 dilution) for 1 hour and then incubated with Alex 488 conjugated donkey anti-Rb IgG (1:500 dilution) for 1 hour and images were taken by Leica fluorescent microscope.
Statistical analysis
Statistical testing was determined by Student’s t-test and P<0.05 was considered as significant.
Results
IFNβ regulates hBMSC proliferation and differentiation
To determine the effect of type I and type II IFN on hBMSC proliferation, hBMSC were treated with IFNα, β or γ (1ng/ml or 100ng/ml) for 9 days. We observed that IFNβ inhibited hBMSC proliferation at day 3 (p<0.001) and there was no difference between the effects of 1 ng/ml or 100 ng/ml IFNβ. The inhibitory effects of IFNα and γ on hBMSC proliferation were first detected at high concentration (100 ng/ml) at day 3 (p<0.001) and day 5 respectively (p<0.05) (Figure 1A). During osteoblast differentiation, IFNα, β or γ (all 1ng/ml) inhibited early osteoblastic differentiation marker ALP’s activity, and among these, IFNβ exhibited the most pronounced effects (Figure 1B). Similarly, IFNβ significantly reduced the steady state gene expression levels of ALP and COL1A1 at day 7 (data not shown) and day 10 (Figure 1C) but not the expression of other osteogenic markers e.g. RUNX2, BSP and osteocalcin (data not shown). In vitro mineralization analysis by using alizarin red staining was not possible in this study due to significant inhibition of cell proliferation by IFNβ. Moreover, no significant inhibitory effects of IFNα and γ on the expression of ALP and COL1A1 were observed (Figure 1C). In contrast, IFNβ increased gene expression of adipocytic markers including CEBPA, PPARG2, AP2, APM1 and LPL (Figure 1D) with more pronounced effects as compared to IFNα and γ. These results demonstrate that IFNβ exerts the most significant effects on hBMSC proliferation and in vitro differentiation.
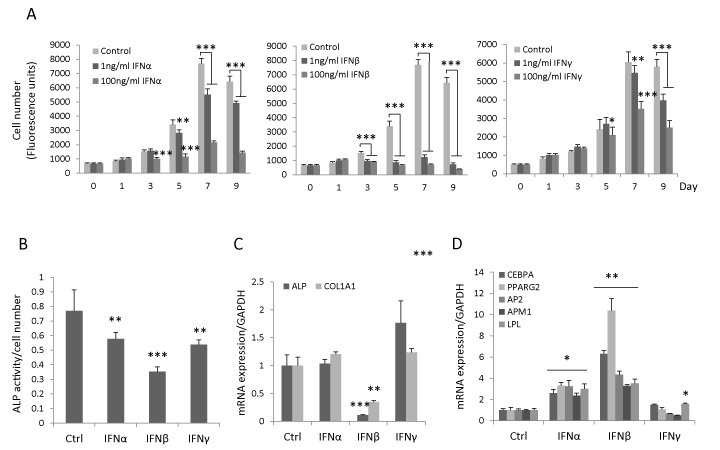
Figure 1: IFNβ inhibits proliferation, osteoblast differentiation and enhances adipocyte differentiation of hBMSC. (A) hBMSC were seeded in 96-well plate and treated with 1ng/ml or 100ng/ml IFNα, β, γ or vehicle control for 9 days. Cell number was determined by CellTiter-Blue reagent (mean±SD, n=8) on the indicated day. (B-D) hBMSC were cultured in osteoblastic/adipocytic induction medium in the presence of 1ng/ml IFNα, β or γ or vehicle control (Ctrl). ALP activity was quantitated at day 7 (mean+SD, n=8) (B). The mRNA expression of osteogenic (C) and adipogenic markers (D) was analyzed by real-time qRT-PCR and normalized against GAPDH at day 10 (mean+SD, n=3-6). * P<0.05, **P<0.01, *** P<0.001 comparing to Ctrl.
IFNβ determines hBMSC differentiation by inhibiting Wnt signaling through Wnt antagonists DKK2
To determine whether IFNβ signaling regulates hBMSC differentiation through interacting with canonical Wnt signaling, we established a stable Wnt luciferase reporter cell line (hBMSCTCF) by sequentially infecting hBMSC with lentivirus expressing renilla luciferase as internal control and lentivirus containing TCF-firefly luciferase reporter construct. By treating hBMSC-TCF cells with IFNα, β or γ (1 ng/ml or 100 ng/ml) in presence of Wnt3a conditioned medium (20%), we observed that all IFNs inhibited Wnt signaling but IFNβ exhibited the most pronounced inhibitory effects and at a lower dose (1ng/ml) (Figure 2A). Concomitantly, IFNβ (1ng/ ml) but not IFNα and γ (1ng/ml) inhibited Wnt3a-induced ALP activity (Figure 2B). In previous studies, we had shown that several components of the Wnt signaling pathway, expressed in hBMSC cells, could be regulated by Wnt3a [14,28,29]. Thus, we determined the effects of IFNβ on these Wnt components by treating hBMSC with IFNβ (1ng/ml) for 3 days in presence of Wnt3a conditioned medium (20%). IFNβ antagonized the effects of Wnt3a treatment on gene expression of Wnt responsive genes. For example, WNT2B and DKK1 [14,29] was downregulated by Wnt3a treatment but was upregulated by IFNβ. Similarly WNT5A, NKD1 and TNFRSF19 [28,29] that were upregulated by Wnt3a treatment, were downregulated by IFNβ (Figure 2C). Unexpectedly, Wnt antagonist DKK2, upregulated by Wnt3a [28], was further up-regulated by IFNβ treatment. The expression of β-catenin (CTNNB1), the key regulator of Wnt signaling, was not changed by IFNβ treatment (Figure 2C). These results demonstrate that the inhibitory effect of IFNβ on Wnt signaling may mediate through Wnt antagonist DKK2.
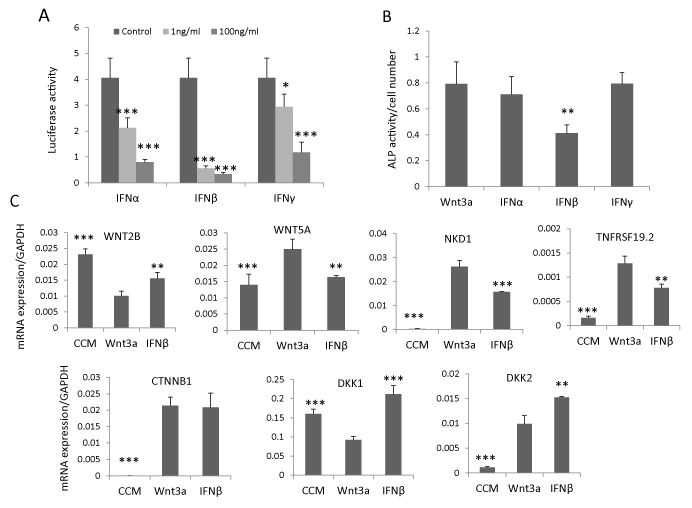
Figure 2: IFNβ inhibits Wnt signaling by upregulation of DKK2. (A) Canonical Wnt luciferase reporter cells hBMSC-TCF were treated with 20% Wnt3a conditioned medium (Wnt3a) as control or Wnt3a plus 1-100ng/ml IFNα, β or γ for 24 hours. Signaling activity was measured by dual luciferase assay (mean+SD, n=5). * P<0.05, *** P<0.001 comparing to control. (B, C) hBMSC was treated with 20% Wnt3a alone or Wnt3a plus 1ng/ml IFNα, β or γ for 7 days for ALP activity quantitation (mean+SD, n=8). ** P<0.01 comparing to Wnt3a. (C) hBMSC was treated with 20% control conditioned medium (CCM), Wnt3a or Wnt3a plus 1ng/ml IFNβ (IFNβ) for three days and mRNA expression was analyzed by real-time qRT-PCR and normalized against GAPDH. (mean+SD, n=3). ** P<0.01, *** P<0.001 comparing to Wnt3a.
The canonical type I IFN signals through type I IFN receptors (IFNAR1 and IFNAR2) and downstream JAK–STAT proteins [4]. To determine whether IFNβ regulated Wnt signaling and hBMSC differentiation through canonical IFN signaling, we blocked the IFNβ signaling by siRNA-based knock down of both IFNAR1 and IFNAR2 receptors (siIFNAR). The knock-down efficiency was determined by type I IFN luciferase reporter cell line (hBMSC-ISRE). siIFNAR reduced the IFNβ activity (Figure 3A), rescued the inhibitory effects of IFNβ on Wnt signaling in hBMSC-TCF cells (Figure 3B), and modestly reduced DKK2 expression (Figure 3C). Additionally, knocking down IFNAR increased ALP activity as well as gene expression of osteoblastic markers (ALP and COL1A1) (Figure 3D, E) and reduced the expression of adipocytic markers (PPPARg2, AP2, APM1 and LPL) (Figure 3F) of hBMSC. These results corroborate that IFNβ regulates hBMSC differentiation through inhibiting Wnt signaling via canonical type I IFN signaling pathway.
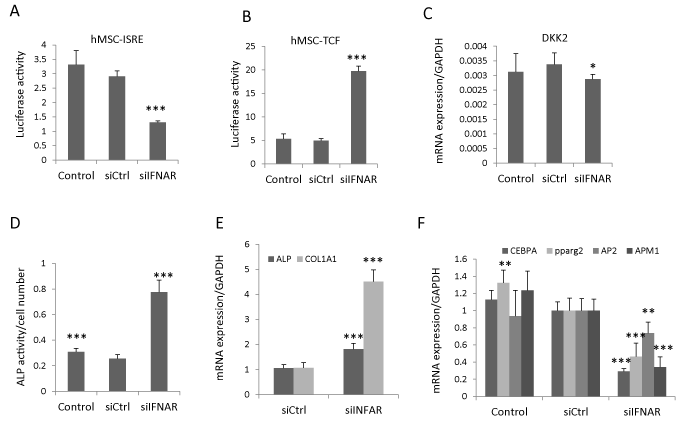
Figure 3: IFNβ inhibits Wnt signaling and hBMSC differentiation by canonical type I IFN pathway. (A,B) Type I IFN luciferase reporter cells (hBMSC-ISRE) or Wnt luciferase reporter cells (hBMSC-TCF) were reverse transfected with control siRNA (siCtrl) or siRNAs targeting IFNAR1 and IFNAR2 (siIFNAR). hBMSC-ISRE cells were treated with 1ng/ml IFNβ to determine knock down efficiency (A) and hBMSC-TCF cells were treated with 20% Wnt3a conditioned medium (Wnt3a) plus 1ng/ml IFNβ for Wnt activity assay (B) (mean+SD, n=6 to 8). (C-E) hBMSC was reverse transfected with siCtrl or siIFNAR followed by treated with 20% Wnt3a plus 1ng/ml IFNβ for three days for DKK2 mRNA expression analysis (C), or followed by osteoblast or adipocyte differentiation in the presence of 1ng/ml IFNβ for 7-10 days. The ALP activity was measured at day 7 (D) and the mRNA expression of differentiation markers was analyzed by real-time qRT-PCR at day 10 and normalized against GAPDH (mean+SD, n=5) (E). In each experiment nontransfected cells with corresponding treatment were used as baseline control. * Pβ0.05, ** P<0.01, *** P<0.001 comparing to siCtrl.
IFNβ determines hBMSC differentiation by inhibiting Wnt signaling through reduced nuclear β-catenin
In addition to its canonical signaling pathway, type I IFN signals through PI3K/AKT pathway [4]. AKT phosphorylates β-catenin at residue Ser552 resulting in a stabilized nuclear-localized active form of β-catenin [30]. The presence of this signaling pathway was demonstrated in hBMSC treated with IFNα, β or γ (1ng/ml). Western blot analysis revealed that phospho-AKT1 was reduced by IFNβ (39% of control) and, to a more pronounced degree, by IFNγ (13% of control) while IFNα exerted no effects (Figure 4A). Correspondingly, IFNβ and γ treatment reduced phosphorylation of β-catenin at Ser552 (44% and 62% respectively) (Figure 4A) and IFNβ reduced nuclear translocation of β-catenin (Figure 4B). Additionally, knocking down AKT1 by siRNA reduced gene expression of osteoblastic markers (ALP, COL1A1, OPN and BSP), and increased the expression of adipocytic markers (PPARG2, AP2 and LPL) (Figure 4C). These results demonstrate that IFNβ regulates Wnt signaling and hBMSC differentiation by reducing the active form of β-catenin through reduced phospho-AKT1 signaling in hBMSC.
(B) hBMSC was treated with 20% Wnt3a conditioned medium (Wnt3a) or Wnt3a with 1ng/ml IFNβ for 2 hours and stained with β-catenin antibody. Images were taken by Leica fluorescence microscopy (Magnification, 200X).
(C) hBMSC were reverse transfected with control siRNA (siCtrl) or siRNA targeting AKT1 (siAKT1) followed by osteoblast or adipocyte differentiation for 10 days. The mRNA expression was analyzed by real-time qRT-PCR and normalized against GAPDH (mean+SD, n=3). Nontransfected cells with corresponding treatment were used as baseline control. The expression level in siCtrl was set to 1 (D). * P<0.05, ** P<0.01, *** P<0.001 comparing to siCtrl.
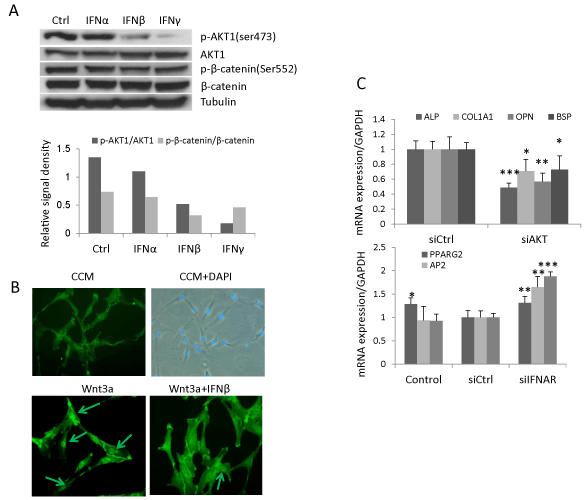
Figure 4: IFNβ regulates Wnt signaling and hBMSC differentiation by reduced AKT1 and β-catenin phosphorylation. (A) hBMSC were treated with 1ng/ml IFNα, β, γ or vehicle as control (Ctrl) for 24 hours for western blot analysis of AKT and β-catenin phosphorylation. Signal density was semi-quantitated by ImageJ software
and normalized against tubulin. Result is the representative of three independent experiments.
(B) hBMSC was treated with 20% Wnt3a conditioned medium (Wnt3a) or Wnt3a with 1ng/ml IFNβ for 2 hours and stained with β-catenin antibody. Images were
taken by Leica fluorescence microscopy (Magnification, 200X).
(C) hBMSC were reverse transfected with control siRNA (siCtrl) or siRNA targeting AKT1 (siAKT1) followed by osteoblast or adipocyte differentiation for 10 days.
The mRNA expression was analyzed by real-time qRT-PCR and normalized against GAPDH (mean+SD, n=3). Nontransfected cells with corresponding treatment
were used as baseline control. The expression level in siCtrl was set to 1 (D). * P<0.05, ** P<0.01, *** P<0.001 comparing to siCtrl.
Discussion
The interaction of immune system with bone cells has been transpired due to the close proximity of immune cells to bone remodeling surfaces, the discovery of an increasing number of immune related cytokines as important regulators of bone cell functions and the deleterious effects of inflammatory diseases e.g. rheumatoid arthritis on the skeleton [1,2]. IFNs are immune factors with important effects on bone cell functions e.g. regulatory effects on osteoclast formation and osteoclastic bone resorption as demonstrated in a number of in vitro and in vivo studies [2,5]. Both IFNAR1-/- and IFNβ-/- mice exhibit a severe osteopenia phenotype along with enhanced osteoclastogenesis [9]. In response to osteoclast activation by RANK-RANKL interaction, IFNβ expression was induced in a c-Fos dependent manner and induced IFNβ, in turn, functioned as inhibitor of c-Fos to inhibit osteoclastogenesis [9]. RANK-RANKL interaction could also induce IFNβ expression through NF-?B which triggers nitric-oxide synthase and nitric oxide as an important negative feedback signal during osteoclastogenesis [31]. IFNβ has additionally been reported to inhibit osteoclastogenesis by upregulation of the chemokine CXCL11 [32]. Another interaction involved in bone homeostasis regulation by IFNβ is the interaction of 4-1BBL and 4-1BB inducing the expression of IFNβ which could be the mechanism underlying the inhibition of osteoclastogenesis by 4-1 BBL signaling [33].
Comparing to the well-studied IFNβ function in osteoclast differentiation, its function in osteoblast differentiation was less understood. Recently, IFNβ was observed to modify osteoblast function by inhibiting extracellular matrix synthesis [10,11]. Our study further demonstrates that IFNβ exerts the most significant effects on hBMSC proliferation and differentiation by interacting with Wnt signaling. We uncovered two possible mechanisms for interaction between IFN and Wnt signaling. First, IFNβ inhibited Wnt signaling by upregulation of DKK2 through canonical IFNβ signaling pathway. Second, IFNβ inhibited Wnt signaling by reducing stable β-catenin through reducing AKT phosphorylation. Bioinformatics analysis of DKK2 promoter did not reveal any IFNβ responsive elements (data not shown) suggesting IFNβ may regulate DKK2 expression indirectly and thus needs further characterized. We also observed that both IFNβ and IFNγ inhibited AKT1 and β-catenin phosphorylation and thus reduced stabilized β-catenin in hBMSC. In contrast, IFNγ was reported to enhance AKT1 and β-catenin phosphorylation upon short-term exposure in intestinal epithelial cells [24]. This discrepancy may be caused by differences in treatment period, antibody for phosphor-AKT or the cells used. IFNα could inhibit Wnt signaling by targeting β-catenin through FOXO3 or RanBP3 [25,26]. However, both FOXO3 and RanBP3 were not expressed or expressed at extremely low levels in our hBMSC cell line (unpublished microarray data), therefore IFNβ may not use these two mechanisms in our cells to regulate Wnt signaling. Our studies were performed in 2D cell culture system, whether IFNβ has similar effect on hBMSC cultured in 3D system need to be explored.
Due to the IFNβ action on bone resorption [2], IFNβ could be a therapeutic target to restore the dysregulated bone homeostasis in many diseases including osteoporosis, rheumatoid arthritis and metabolic cancer. However, a placebo controlled clinical phase II study of IFNβ in the treatment of patients with active rheumatoid arthritis failed to show an improvement in radiological scores or biomarkers of bone resorption [34]. Clinically, IFNβ has approved treatment for the relapsing forms of multiple sclerosis, which has a high risk of low bone density [35]. In an open-label pharmacodynamics study, IFNβ treatment has shown to induce complex, specific and time-dependent changes in the expression of markers of both bone resorption and bone formation [36]. However, bone mineral density could not be measured in this study. Thus far, two studies have been performed to investigate the effect of IFNβ treatment on bone mineral density in multiple sclerosis. One study showed that IFNβ favored bone mineral density [37] but another study showed no effect on bone mineral density [38]. Together with our data, IFNβ could be a therapeutic target for bone diseases but its effects on both bone formation and bone resorption should be considered.
Conclusion
In conclusion, we uncovered novel role for the IFNβ signaling in controlling the differentiation fate of MSC into osteoblasts versus adipocytes through inhibiting Wnt signaling. Targeting IFNβ signaling in MSC is therefore a possible approach for controlling MSC differentiation and enhancing bone formation.
Acknowledgement
We thank Linda Harkness for proof reading. This work was supported by grants from Fabrikant Vilhelm Pedersen og Hustrus, the Novo Nordisk Foundation and a local grant from Odense University Hospital.
Supplementary Table
Primer Name
Forward primer (5’ to 3’)
Reverse primer (5’ to 3’)
ALP
ACGTGGCTAAGAATGTCATC
CTGGTAGGCGATGTCCTTA
COL1A1
AGGGCTCCAACGAGATCGAGATCCG
TACAGGAAGCAGACAGGGCCAACGTCG
OPN
CCAAGTAAGTCCAACGAAAG
GGTGATGTCCTCGTCTGTA
BSP
GATTTCCAGTTCAGGGCAGT
TCCTCTCCATAGCCCAGT GT
CEBPA
CACGAAGCACGATCAGTCC
CATTGCACAAGGCACTGC
PPARG2
TTCTCCTATTGACCCAGAAAGC
CTCCACTTTGATTGCACTTTGG
APM1
TGTTGCTGGGAGCTGTTCTACTG
ATGTCTCCCTTAGGACCAATAAG
AP2
GCCAGGAATTTGACGAAGTC
TGGTTGATTTTCCATCCCAT
LPL
GAGATTTCTCTGTATGGCACC
CTGCAAATGAGACACTTTCTC
WNT2B
TCATGCTCAGAAGTAGCCGAGA
TGGCACTTACACTCCAGCTTCA
WNT5A
TTTTTCTCCTTCGCCCAGGTTGT
GGCTCATGGCGTTCACCAC
NKD1
GATGGAGAGAGTGAGCGAACC
CATAGATGGTGTGCAGCAAGC
TNFRSF19.2
CATTTCATCTCCCTGCTCG
GCCACATTCCTTAGACAACTCC
CTNNB1
GCTGATTTGATGGAGTTGGACATGG
GCCAAACGCTGGACATTAGTGG
DKK1
TTCCAGCGTTGTTACTGTGG
AATAGGCAGTGCAGCACCTT
DKK2
AGCATCTTAACCCCTCACATCC
TTTCCAGCCCATGAGAACC
GAPDH
GGCGATGCTGGCGCTGAGTAC
TGGTTCACACCCATGACGA
Table 1: Primers used in this study.
References
- Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007; 7: 292-304.
- Takayanagi H, Sato K, Takaoka A, Taniguchi T. Interplay between interferon and other cytokine systems in bone metabolism. Immunol Rev. 2005; 208: 181-193.
- Goodbourn S, Didcock L, Randall RE. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J Gen Virol. 2000; 81: 2341-2364.
- Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005; 5: 375-386.
- Abraham AK, Ramanathan M, Weinstock-Guttman B, Mager DE. Mechanisms of interferon-beta effects on bone homeostasis. Biochem Pharmacol. 2009; 77: 1757-1762.
- Duque G, Huang DC, Dion N, Macoritto M, Rivas D, Li W, et al. Interferon-? plays a role in bone formation in vivo and rescues osteoporosis in ovariectomized mice. J Bone Miner Res. 2011; 26: 1472-1483.
- Duque G, Huang DC, Macoritto M, Rivas D, Yang XF, Ste-Marie LG, et al. Autocrine regulation of interferon gamma in mesenchymal stem cells plays a role in early osteoblastogenesis. Stem Cells. 2009; 27: 550-558.
- Vidal C, Bermeo S, Li W, Huang D, Kremer R, Duque G. Interferon gamma inhibits adipogenesis in vitro and prevents marrow fat infiltration in oophorectomized mice. Stem Cells. 2012; 30: 1042-1048.
- Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, et al. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature. 2002; 416: 744-749.
- Woeckel VJ, Eijken M, van de Peppel J, Chiba H, van der Eerden BC, van Leeuwen JP. IFNß impairs extracellular matrix formation leading to inhibition of mineralization by effects in the early stage of human osteoblast differentiation. J Cell Physiol. 2012; 227: 2668-2676.
- Woeckel VJ, Koedam M, van de Peppel J, Chiba H, van der Eerden BC, van Leeuwen JP. Evidence of vitamin D and interferon-ß cross-talk in human osteoblasts with 1a,25-dihydroxyvitamin D3 being dominant over interferon-ß in stimulating mineralization. J Cell Physiol. 2012; 227: 3258-3266.
- Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013; 19: 179-192.
- Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006; 98: 251-266.
- Qiu W, Andersen TE, Bollerslev J, Mandrup S, Abdallah BM, Kassem M. Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J Bone Miner Res. 2007; 22: 1720-1731.
- Baron R. Wnt signaling, LRP5 and gut serotonin: Have we been targeting the right pathway for the wrong reasons? IBMS BoneKEy. 2009; 6: 86-93.
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001; 107: 513-523.
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002; 346: 1513-1521.
- Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002; 70: 11-19.
- Van Wesenbeeck L, Cleiren E, Gram J, Beals RK, Bénichou O, Scopelliti D, et al. Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am J Hum Genet. 2003; 72: 763-771.
- Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005; 102: 3324-3329.
- Morvan F, Boulukos K, Clément-Lacroix P, Roman Roman S, Suc-Royer I, Vayssière B, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006; 21: 934-945.
- Bain G, Müller T, Wang X, Papkoff J. Activated beta-catenin induces osteoblast differentiation of C3H10T1/2 cells and participates in BMP2 mediated signal transduction. Biochem Biophys Res Commun. 2003; 301: 84-91.
- Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005; 132: 49-60.
- Nava P, Koch S, Laukoetter MG, Lee WY, Kolegraff K, Capaldo CT, et al. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity. 2010; 32: 392-402.
- Parody JP, Alvarez ML, Quiroga AD, Ceballos MP, Frances DE, Pisani GB, et al. Attenuation of the Wnt/beta-catenin/TCF pathway by in vivo interferon-alpha2b (IFN-alpha2b) treatment in preneoplastic rat livers. Growth Factors. 2010; 28: 166-177.
- Thompson MD, Dar MJ, Monga SP. Pegylated interferon alpha targets Wnt signaling by inducing nuclear export of ß-catenin. J Hepatol. 2011; 54: 506-512.
- Simonsen JL, Rosada C, Serakinci N, Justesen J, Stenderup K, Rattan SI, et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002; 20: 592-596.
- Qiu W, Hu Y, Andersen TE, Jafari A, Li N, Chen W, et al. Tumor necrosis factor receptor superfamily member 19 (TNFRSF19) regulates differentiation fate of human mesenchymal (stromal) stem cells through canonical Wnt signaling and C/EBP. J Biol Chem. 2010; 285: 14438-14449.
- Qiu W, Chen L, Kassem M. Activation of non-canonical Wnt/JNK pathway by Wnt3a is associated with differentiation fate determination of human bone marrow stromal (mesenchymal) stem cells. Biochem Biophys Res Commun. 2011; 413: 98-104.
- He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007; 39: 189-198.
- Zheng H, Yu X, Collin-Osdoby P, Osdoby P. RANKL stimulates inducible nitric-oxide synthase expression and nitric oxide production in developing osteoclasts. An autocrine negative feedback mechanism triggered by RANKL-induced interferon-beta via NF-kappaB that restrains osteoclastogenesis and bone resorption. J Biol Chem. 2006; 281: 15809-15820.
- Coelho LF, Magno de Freitas Almeida G, Mennechet FJ, Blangy A, Uzé G. Interferon-alpha and -beta differentially regulate osteoclastogenesis: role of differential induction of chemokine CXCL11 expression. Proc Natl Acad Sci U S A. 2005; 102: 11917-11922.
- Shin HH, Lee EA, Kim SJ, Kwon BS, Choi HS. A signal through 4-1BB ligand inhibits receptor for activation of nuclear factor-kappaB ligand (RANKL)-induced osteoclastogenesis by increasing interferon (IFN)-beta production. FEBS Lett. 2006; 580: 1601-1606.
- van Holten J, Pavelka K, Vencovsky J, Stahl H, Rozman B, Genovese M, et al. A multicentre, randomised, double blind, placebo controlled phase II study of subcutaneous interferon beta-1a in the treatment of patients with active rheumatoid arthritis. Ann Rheum Dis. 2005; 64: 64-69.
- Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG) Ann Neurol. 1996; 39: 285-294.
- Weinstock-Guttman B, Hong J, Santos R, Tamaño-Blanco M, Badgett D, Patrick K, et al. Interferon-beta modulates bone-associated cytokines and osteoclast precursor activity in multiple sclerosis patients. Mult Scler. 2006; 12: 541-550.
- Shuhaibar M, McKenna MJ, Au-Yeong M, Redmond JM. Favorable effect of immunomodulator therapy on bone mineral density in multiple sclerosis. Ir J Med Sci. 2009; 178: 43-45.
- Varoglu AO, Varoglu E, Bayraktar R, Aygul R, Ulvi H, Yildirim K. The effect of interferon beta 1B on bone mineral density in multiple sclerosis patients. J Back Musculoskelet Rehabil. 2010; 23: 25-29.