Research Article
Austin J Pharmacol Ther. 2014; 2 (5). 1029
Evaluation of in vivo Antitrypanosomal activity of aqueous and methanol leaf extracts of Clutia abyssinica (Euphorbiaceae) against Trypanosoma congolense
Mergia E1, Terefe G2, Teklehaymanot T3 and Shibeshi W1*
1Department of Pharmacology and Clinical Pharmacy, Addis Ababa University, Ethiopia
2Department of Parasitology and Pathology, Addis Ababa University, Ethiopia
3Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Ethiopia
*Corresponding author: : Shibeshi W, Department of Pharmacology and Clinical Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Received :June 19, 2014; Accepted: July 31, 2014; Published: Aug 04, 2014
Abstract
Introduction: African trypanosomiasis is a major disease of economic and public health importance affecting agricultural and human development. The search for alternative compounds against African trypanosomiasis is essential as existing chemotherapeutic agents have several drawbacks. The objective of the study reported was to evaluate aqueous and methanol leaf extracts of Clutia abyssinica for in vivo activity in Trypanosoma congolence infected mice.
Materials and Methods: The in vivo antitrypanosomal efficacy of the aqueous and methanol extracts was evaluated in Swiss albino mice infected with T. congolense isolated from naturally infected cattle. The leaf extracts were administered 12 days post-infection at doses of 100, 200 and 400 mg/kg by intraperitoneal injection once daily for 7 days. Parasitaemia, packed cell volume (PCV), mean survival time and change in body weight were used as indices for monitoring the efficacy of the extracts by comparing with the positive control (28 mg/kg dose of diminazene aceturate) and negative control (2% tween 80) treated groups.
Results: Highly significant (p<0.001) reduction in pre-treatment parasitaemia by 3.91% (7.38 ± 0.18), increase in PCV by 1.12% (48.66 ± 0.20), body weight improvement by 1.36% (22.34 ± 0.27) and mean survival time of 39.20 ± 0.37 days was observed in the group treated with 400 mg/kg methanol leaf extract of C. abyssinica on day 14 of treatment while the mice treated with aqueous extracts of C.abyssinica at 400 mg/kg dose had low parasitaemia on day 6 (p<0.01), day 8, 10 and 14 (p<0.001) of treatment as compared to the negative control.
Conclusion: The results obtained confirm ethno-pharmacological usefulness of the plant in treatment of trypanosomiasis and possible indications in development of alternative drugs.
Keywords: Clutia abyssinica; In vivo antitrypanosomal activity; Mice; Trypanosoma congolense
Abbreviations
T. congolense: Trypanosoma Congolense; C. abyssinica: Clutia Abyssinica; CAAE: C. Abyssinica Aqueous Extract; CAME= C. Abyssinica Methanol Extract; DA: Diminazine Aceturate
Introduction
African trypanosomosis is a protozoan disease of humans and livestock caused by trypanosomes and transmitted by tsetse fly vector. While Trypanosoma rhodiense and Trypanosoma gambiense cause African Human Trypanosomosis, Trypanosoma brucei brucei, Trypanosoma vivax, Trypanosoma congolense, Trypanosoma evansi and Trypanosoma equiperdum are the agents of African Animal Trypanosomosis (AAT) [1].
The economic impacts of trypanosomosis in Africa are diverse and complex, with direct effects on animal production and human health, as well as indirect effects on settlement patterns, land use, animal husbandry and farming [2]. Chemotherapy, the main means of controlling the disease is under threat due to parasite resistance [3] and toxicity of the trypanocidal drugs [4]. The poor prospect for a vaccine due to antigenic variation of the parasite is further compounded by unwillingness of the pharmaceutical industry to develop new compounds because of uncertain and unprofitable market or perhaps the localized nature of the disease.
The few commercial trypanocides (diminazene aceturate, isometamidium and homidium) have been in use for well over 40 years. Thus, the search for medicinal plants with trypanocidal activities continues to generate a lot of research interest [5,6]. Although recent reports indicate antitrypanosomal activity exists in some medicinal plants [7-11], the potentials of many other plants used in folkloric medicine in Ethiopia are yet to be investigated. Euphorbiaceae is a large and fascinating family of about 300 genera and 8,000-10,000 species, mostly found in the tropics of both hemispheres.
Clutia is a genus within a family Euphorbiaceae, having about 60 species. Clutia abyssinica called by the Amharic name ‘fyele fej’ is herb 1-2 m high [12]. Traditionally it is used in treatment of venereal and skin diseases, chest problems, cancer [13]; Skin fungal infections [14,15]; yellow fever and malaria; management of ear, nose and throat diseases [16]; diarrhoea [17]; gonorrhea, cough and fever, headache, toothache, menstrual pain, burns, pneumonia, enlarged spleen and kidney, shock, abdominal problems- as a laxative and to expel intestinal worms, elephantiasis, diarrhoea and tachycardia [15]. The maceration of the crushed leaves of C. abyssinica given orally has been traditionally used for the treatment of animal trypanosomosis [18]. Therefore, this study was aimed to evaluate the in vivo antitrypanosomal activity of Clutia abyssinica in mice infected with T. congolense isolated from natural infection of cattle.
Materials and Methods
Chemicals and drugs
The chemical reagents used were diminazine aceturate (Ceva Santé Animale, France; batch number- 719A1), Giemsa stain, tween-80 (BDH Ltd, England), 40% glucose (Pharmacure, Ethiopia), methanol (Carlo ebra reagents, Italy).
Test organism
The test organism T. congolense was isolated from infected cattle in Sebategna kebele, Bedele town, Dabo Hana woreda, 480 km south west of Addis Ababa. The presence of T. congolense in the screened cattle was detected from blood samples collected from the ear vein of the animals. The slide was examined for T. congolense based on their type of motility in the microscopic field 40x objective and confirmations of T. congolense species by morphological characteristics was done by staining with Giemsa stain, and examination under a microscope using oil immersion 100x objectives [19,20]. Then the infected blood was collected from the jugular vein of the animal using EDTA coated tubes and heavily inoculated to laboratory mice and transported to laboratory at Akililu Lemma Institute of Pathobiology, Addis Ababa University for subsequent serial passage to other mice.
Experimental animals
Healthy Swiss albino mice (weighing 20–30 gm and age of 8–12 weeks) were obtained from the animal house of the Ethiopian Health and Nutrition Research Institute and School of Pharmacy, Addis Ababa University. Animals were housed in polypropylene cages (6–10 animals per cage), maintained less than 12 hr light and 12 hr dark cycle and allowed free access to pellet diet and clean water ad libitum. All procedures complied with the guide for the care and use of laboratory animals [21]. The experimental protocols and use and handling of animals were approved by research and ethics committee of Department of Pharmacology and clinical pharmacy.
Preparation of plant materials
The leaves of C. abyssinica were collected from Debre Libanos Monastery in Amhara regional state, Ethiopia. The fresh leaves were wrapped by plastic sheets during transportation. Taxonomic identification was done and a voucher specimen was deposited (Collection EM/001) at the National Herbarium, College of Natural sciences, and Addis Ababa University. The leaves were washed with distilled water and were dried under shade. The dried leaves were pulverized using mortar and pestle. For preparation of extracts, 200g of dried leaf powder of C. abyssinica was separately macerated with 1000 ml of distilled water and absolute methanol for 48 hours with frequent agitation in orbital shaker and the resulting liquid was filtered using Whatman No. 3 filter paper (Whatman Ltd., England). Extraction was repeated three times and the filtrates of all portions were pooled in one vessel. The aqueous extract was placed in a petridish and lyophilized for one week to yield a solid residue, while the methanol extract was concentrated using Rota vapor (BÜCHI Rota-vapor, Switzerland) at no more than 40°C in order to obtain dry extract. Then the resulting dried mass was then powdered and weighed resulting in yield of 12.92% and 17.21% for aqueous and methanol extracts respectively.
Acute toxicity study
The acute toxicity study was conducted in accordance with the Lorke’s [22] method. The study was conducted for each extract in two phases using female Swiss albino mice after 7 days of adaptation. In the first phase, nine mice were divided into 3 groups (n=3). Each group was given 10, 100, and 1000 mg/kg body weight of the test substance respectively. In the second phase, further specific doses (1600, 2900, and 5000 mg/kg) of each extract were administered to nine mice to estimate the lethal dose (LD50) value. The control group received the reconstituting solvent 2% tween 80 in sterile water. The extract was dissolved in 2% tween 80 in sterile water and given through intraperitoneal route. All animals were kept under strict observation for behavioral, neurological, autonomic or physical changes such as alertness, motor activity, restlessness, convulsions, coma, diarrhea and lacrimation for 24 h. These observations continued for further 14 days for any signs of overt toxicity. Then the lowest dose which killed one mouse (minimum toxic dose) and the highest dose which had not killed any mouse (maximum tolerated dose) were noted, and the geometric mean of these two doses gave LD50. The detailed procedures for determining the doses for testing acute toxicity are shown in annex 1.
Parasite inoculation and extract administration
Healthy Swiss albino mice that are infected intraperitoneally with 0.2 ml of T. congolense infected blood (~104 trypanosomes/ ml) collected by cardiac puncture from donor mice was divided into eight groups (n =5): C. abyssinica aqueous extract (CAAE 100, CAAE 200, CAAE 400), C. abyssinica methanol extract (CAME 100, CAME 200, CAME 400), diminazene aceturate (DA28), and 2% tween 80 (TW80). Treatment with the extracts began on the 12th day post-infection (day 0 of treatment), when the infected mice showed peak parasitaemia of (~108 trypanosomes/ml). On each day of drug administration, the aqueous and methanol extracts of C. abyssinica were freshly prepared by dissolving in 2% Tween-80 in sterile water for injection and administered intraperitoneally daily at 9 am for seven days at the doses of (100, 200 and 400 mg/kg). The doses were selected based on the acute toxicity study. For the positive control, diminazine aceturate (DA28), dissolved in sterile water as recommended by the manufacturer was administered at the dose of 28 mg/kg intraperitoneally based on previous reports of Moti et al. [23], Feyera et al. [11], while for the negative control, 2% tween 80 in sterile water (TW80), was administered intraperitoneally. Volume administered was 2 ml/100 gm of body weight of the animal (OECD, 2001).
Determination of parasitaemia
Parasitaemia was monitored every other day by microscopic examination of blood obtained from the tail of each mouse that was pre-sterilized with methylated spirit. The tail tip was cut to extrude blood and drop of blood was placed on microscope slide and covered with a cover-slide. The blood was examined microscopically at 400x total magnification. The degree of parasitaemia was determined using the “Rapid Matching” method of Herbert and Lumsden [24]. Wet smear were prepared in triplicates from each animal and the mean value of slide counts were taken per sample examined microscopically. Logarithm values of these counts were obtained by matching with the table given by Herbert and Lumsden [24].
Determination of packed cell volume (PCV)
PCV was measured [25] to predict the effectiveness of the test extracts in preventing hemolysis resulting from increasing parasitaemia associated with trypanosomosis. It was monitored before infection and three times till the 14th day (on day 0, 7 14). Briefly, blood was collected from tail of each mouse in heparinized microhaematocrit capillary tubes filled up to 3/4th of their length. The tubes were then sealed immediately by cristal seal and centrifuged in a microhaemtocrit centrifuge (Hettich Haematokrit, Germany) for 5 min at 12,000 rpm. After centrifugation, the height of the red blood cell column were measured by use of haematocrit reader and compared to the total height of the column of the whole blood [26]. The effect of extracts in improving PCV of treated animals was compared with the controls.
Determination of body weight
The body weight of each mouse in all groups was measured before infection, on the day treatment commenced (day 0) and every other day up to day 14.
Determination of mean survival time
Mortality was monitored daily and the number of days from the time of inoculation of the parasite up to death was recorded for each mouse in the treatment and control groups throughout the follow up period for six weeks.
Statistical analysis
Values of the data obtained from the study were summarized and expressed as mean ± standard error of mean (SEM). Data analysis was performed using Statistical Package for Social Science (SPSS), version 17.0. To compare the results obtained from different groups, one way ANOVA followed by Tukey’s multiple comparison tests were performed to determine statistical significance. P values less than 0.05 were considered significant.
Results
Acute toxicity test
The acute toxicity bioassay had shown that the lethal dosage (LD50) of the aqueous and methanol leaf extracts of C. abyssinica was above 2000 mg/kg and there were no evidence of acute toxicity at the doses tested indicating good safety margin. As shown on annex 1, the lethal dosage (LD50) for aqueous and methanol extract of C.abyssinica were 2154.065 mg/kg and 3807.886 mg/kg body weight respectively.
Effect of extracts on parasitaemia
Mice treated with aqueous extract of C. abyssinica at 400 mg/ kg dose had low parasitaemia on day 6 (p<0.01), day 8, 10 and 14 (p<0.001) (Table 1), while mice treated with the methanol extract at 200 and 400 mg/kg dose had statistically significant low parasitaemi on day 6, 8, 10 and 14 (p<0.001) of treatment as compared to the negative control group (Table 2). Comparison of the percentage change in parasitaemia on day 14-0 showed that only 400mg/kg dose of the methanol extract significantly (p<0.05) reduced parasitaemia by 3.91%. The lowest mean parasitaemia value (5.94+0.24) was observed in the same group on day 8 of treatment which was highly significant (p<0.001) when compared to the 100 and 200mg/kg dose. Animals treated with diminazene have cleared the parasites from circulation though parasitemia relapsed as of day 12 of treatment.
Effect of extracts on packed cell volume
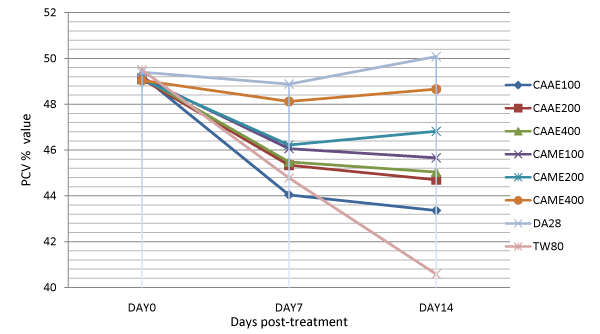
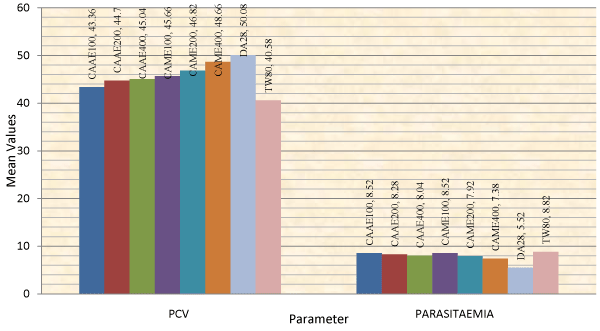
The animals treated with 400 mg/kg of the aqueous extract of C. abyssinica, and with 200 and 400 mg/kg dose of the methanol extract had significantly (p<0.001) higher PCV value as compared to the negative control groups on day 14 of treatment (Table 3). Analysis of change in percentage of PCV from day 7 to day 14 of treatment also showed that the methanol extract at 200 and 400 mg/kg doses had significantly increased PCV value of treated animals by 1.29 and 1.12%, respectively as compared to the negative control groups which had a drop in PCV by 9.38 % from day 7 to 14 of treatment (Table 4). This finding was consistent with the effect shown by the extracts on parasitaemia of T. congolense infected mice (Figure 2). Animals treated with diminazene showed significantly improved PCV compared to all groups at days 7 and 14 of treatment (Figure 1).
Figure 1: Comparison of the effect of aqueous and methanol leaf extracts of Clutia abyssinica on packed cell volume of Trypanosoma congolense infected mice.
Figure 2: Comparison of the effect of aqueous and methanol leaf extracts of Clutia abyssinica on packed cell volume and parasitaemia.
Effect of extracts on body weight
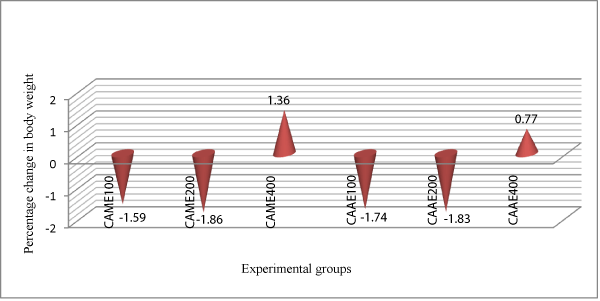
Animals treated with 400 mg/kg dose of the aqueous extract of C.abyssinica had shown significant improvement on their body weight on day 6 (p<0.01), days 8-14 (p<0.001) of treatment, while animals treated with the methanol extract at 400 mg/kg dose had improved body weight (p<0.001) as compared to the negative control group and lower dose (100 and 200 mg/kg) treated groups at p<0.05. In addition animals treated with 400 mg/kg dose of the methanol extract had shown an improvement in their body weight by 1.36% (Figure 3).
Figure 3: Comparison of the effect of aqueous and methanol leaf extracts of Clutia abyssinica on percentage change in body weight of Trypanosoma congolense infected mice.
Effect of extracts on mean survival time
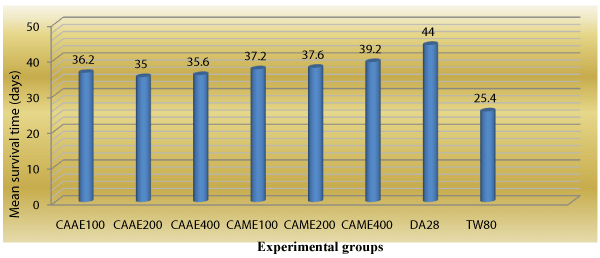
Animals treated with 400 mg/kg of the methanol extract of C. abyssinica had highest mean survival time of 39.20±0.37 days as compared to the negative control group (25.40 ± 0.43) (Table 5), while animals that received the standard drug (diminazine aceturate) had mean survival time of 44.00 ± 0.63 days (Figure 4).
Figure 4: Survivality of Trypanosoma congolense infected mice treated with aqueous and methanol crude extracts of leaves of Clutia abyssinica.
Discussion
Since the few trypanocides developed over 40 years a go are expensive and toxic [4], it has become necessary to search for new compounds that are safe and efficacious, especially those of plant origin. The plant screened in the present study has folkloric medicinal uses as fever remedies and treatment of infectious diseases including trypanosomosis [17,18]. Based on this, the aqueous extract was prepared by macerating the dried leaves in distilled water in order to simulate the way it is traditionally used [17,18]. With the assumption that some of the active ingredients responsible for the claimed antitrypanosomal activity might not be soluble in water adequately; the methanol leaf extract of the plant was also included in the study. Based on the results of the acute toxicity study, the plant extracts had shown LD50 greater than 2000 mg/kg. Thus, since C. abyssinica is believed to have several traditional medicinal uses by different traditional healers, the experimental determination of this good safety margin would justify that the plant is safe at the dose levels (100, 200 and 400 mg/kg) used in the study which is an additional proof for the medicinal value of the plant in folk medicine, as toxic plants will not be used for generations.
According to the results of the phytochemical screening study, the methanol extract of C. abyssinica showed positive test for the presence of alkaloids, flavonoids, glycosides, steroids, tannins and terpenes, while the aqueous extract showed a positive result only for anthraquinones, phenolic compounds and polyphenols (unpublished report).
Numerous in vivo studies conducted on the antitrypanosomal activities of the class of compounds listed above reported the potential of each class of compounds in killing or inhibiting the growth of wide ranges of trypanosomes. Inhibition of the trypanosome alternative oxidase (TAO) enzyme was thought to be responsible for antitrypanosomal activity of phenolic compounds [27]. Quininos can induce oxidative stress in trypanosomes (T. congolense and T. cruzi). This may be explained by their reduction to semiquinone radicals by enzymes such as those present in the mitochondrial electron transport chain and the trypanothione reductase [28]. Flavonoids and flavonoid-derived plant natural products have long been known to function as free radical scavengers and metal chelators which inhibit lipid peroxidation [29].
One of the molecular actions of tannins is by complexing proteins through the so-called nonspecific forces such as hydrogen bonding and hydrophobic effects, as well as by covalent bond formation [30]. The proposed mechanism for antitrypanosomal activity of terpenes include formation of aldehyde-thiol adducts with sulphur containing components thereby decreasing the buffering agents which can create oxidative stress in cells [31]; oxidation of glutathione, pyruvic and alpha-ketoglutaric acids and the oxidative decarboxylation of pyruvic acid by hydroperoxy group which makes them toxic [32]. Therefore, the observed antitrypanosomal activity of C. abyssinica might be attributed to either the individual class of compounds, or to the synergistic effect that each class of compounds exerts to give the observed biological activity. Hence, further in-depth investigations should be carried out to resolve this issue [33,34].
The results obtained during the monitoring period had shown that the higher dose (400mg/kg) of the methanol extracts of C. abyssinica exhibited significant antitrypanosomal activity by reducing the level of parasitaemia by 3.91% as compared to the 18.55% increment in the negative control group and prolonging the lifespan of the test animals beyond that of the negative control by more than 10 days (Table 4). This trypanostatic effect might correlate with the type of antiviral activity reported by Colegate and Molyneux [35], and Cos et al. [36]. In that report, it was shown that the ethanolic leaf, stem and root extracts of C. abyssinica showed moderate antiviral activity against polio virus and Coxsackie virus [35], while the leaf extracts exhibited anti-HIV-1 activity [36].
Although we do not yet know the mechanism by which the extract exerts this remarkable trypanostatic effect, our speculation, based on current literature on mechanisms of anti-trypanosomal compounds, is that the extract may be interfering with cell cycle progression in the parasite, possibly causing cell cycle arrest and thereby halting cell proliferation, which is a similar mechanism for currently available antitrypanosomal agents. It is also possible that the extract might be exerting its trypanostatic effect through the modulation of the animal’s immune system, which in turn enables the animal to withstand the ravaging parasites for a long time. It may well be the interplay of both effects that resulted in the observed tremendous trypanostatic effect.
In addition, the findings of this study had shown that the plant extracts did not completely eliminate parasites from the blood stream of infected mice, but only reduced the level of parasitaemia. Several researchers made similar observations on reduction in parasitaemia [7,8,37]; and concluded that high parasite load could mask the efficacy of crude extract [38]. The reduced efficacy of the crude extracts in clearing trypanosomes from blood circulation could be due to enzymatic inactivation of active compounds and impaired absorption from the site of administration [38]. In addition, failing to reach target organs in sufficient concentration and duration to effect a cure; short half life of the constituents making them unable to stay long enough to exert pronounced effect on the parasites [39] could also be attributed to the failure of the extracts to clear the blood of infected mice from trypanosomes.
In the group of mice treated with diminazine aceturate, there was no parasite development from day 2 to 10 although relapse occurred in all mice approximately on days 12-14 of treatment. Similar observations were made by Afewerk et al. [40], Assefa and Abebe [41], Delespaux et al. [42], Ibrahim et al. [8], Miruk et al. [43]. The relapse of parasitaemia might be due to drug resistance by trypanosomes or the ability of T. congolense to sequester in small vessels and capillaries of the heart, skeletal and other tissues [44,45,46].
The study on packed cell volume (PCV) gave results that were fairly consistent with the observations made on parasitaemia (Figure 2). Infection caused significant drop in PCV in the negative control groups approximately by day 25 post-infection (14th day of treatment) with mean value being below the reference values (42-52%). The low PCV value in the infected groups may be due to acute hemolysis and is a result of the growing infection. In addition infection with trypanosomes results in increased susceptibility of red blood cell membrane to oxidative damage. Reactive oxygen species generated by trypanosomes can also attack red blood cells’ membranes, induce oxidation and subsequently hemolysis. This phenomenon subjects RBC to massive erythrophagocytosis by an expanded and active mononuclear phagocytic system of the host resulting in anemia [47].
The effect of extracts in ameliorating anemia is possibly by reducing the parasite load, neutralizing the toxic metabolites produced by trypanosomes or scavenging the trypanosome associated free radicals which could be attributed to the secondary metabolites present in the extracts [48-50]. The infected mice treated with the diminazine aceturate showed significant improvement in PCV. This is because the drug was able to eliminate parasites from the blood to levels detectable by microscopy on days 2-10. This is in harmony with previous reports [51,52].
The trypanosuppresive effect of the extracts against trypanosome infection can further be inferred from the weight status of the treated animals. At day 14 post-treatment, animals that received 400 mg/kg dose of the aqueous and methanol extracts of C. abyssinica gained weight by 0.77% and 1.36%, respectively (Tables 3 & 4) at (p<0.001) as compared to the negative control groups. This shows that as a result of reduction in parasitaemia and prevention of drop in PCV by the extracts and physical status of the treated mice improved. They were therefore more able to resist weight loss that is usually associated with trypanosomosis. Similar observations have been made by other researchers [51-55]. Based on our findings, animals in the negative control group lost 11.03% of their body weight which might be due to the significant drop in PCV associated with high level of parasitaemia.
The majority of trials provide evidence of the negative effect of trypanosomosis on body weight. During the high levels of parasitaemia the appetite is decreased and the animal losses condition resulting in wasting. There is consumption of the fat reserves but there are also severe degenerative changes of the muscle cells and other tissue cells, and there is an increased breakdown of protein in muscles and elsewhere leading to atrophic degeneration. The decreased supply of oxygen because of the anemia is also an important factor [56,57].
Conclusion
This study evaluated the in vivo antitrypanosomal activity of aqueous and methanol crude leaf extracts of C. abyssinica against T. congolence. The aqueous extract exhibited lower in vivo antitrypanosomal activity, while the methanol extracts have shown better activity at higher doses. The methanol leaf extract of C. abyssinica has promising effect by reducing pre-treatment parasitemia, increasing PCV, increasing pretreatment body weight and prolonging survival time in T. congolense infected mice. Generally, the current study established that leaves of C. abyssinica could have potential antitrypanosomal activity which can be considered as a potential source for the search of new drugs against Africal animal trypanosomosis.
Acknowledgement
We would like to thank staffs of the National Tsetse and Trypanosomosis Investigation and Control Center for technical assistance during isolation of the test organism; Vice President for research and Technology transfer of Addis Ababa university for partly funding the research under the framework of the thematic research project, Animal Health Improvement; and staffs of Aklilu Lemma Institute of Pathobiology, Addis Ababa university for their cooperation and unreserved help during the laboratory work.
References
- Getachew A. Trypanosomosis in Ethiopia. Journal of Biological Sciences. 2000; 4: 75-121.
- Chanie M, Adula D, Bogale B. Socio-Economic assessment of the impacts of trypanosomiasis on cattle in Girja district, Southern Oromia Region, Southern Ethiopia. Acta Parasitologica Globalis. 2013; 4: 80-85.
- Chitanga S, Marcotty T, Namangala B, Van den Bossche P, Van Den Abbeele J, Delespaux V, et al. High prevalence of drug resistance in animal trypanosomes without a history of drug exposure. See comment in PubMed Commons below PLoS Negl Trop Dis. 2011; 5: e1454.
- Amaechi N. Toxicity of antiprotozoan drug, diminazene aceturate in rats. Journal of Sustainable Agriculture and Environment. 2001; 3: 365-370.
- Hoet S, Opperdoes F, Brun R, Quetin-Leclercq J. Natural products active against African trypanosomes: a step towards new drugs. See comment in PubMed Commons below Nat Prod Rep. 2004; 21: 353-364.
- Sampson W. Studying herbal remedies. See comment in PubMed Commons below N Engl J Med. 2005; 353: 337-339.
- Wurochekke AU, Nok AJ. In vitro and in vivo antitrypanosomal activity of the leaf of Lawsonia inermis against Trypanosoma brucei brucei infection in mice. Journal of Medical Sciences 2004; 4: 236-239.
- Ibrahim H, Ogbadoyi E, Adamu K, Bello M, Yemisi I. Evaluation of antitrypanosomal activity of ethyl acetate extract of Adansonia digitata seed extract in T. b. brucei infected albino mice. International Journal of Drug Research and Technology. 2012; 2.
- Shuaibu MN, Wuyep PT, Yanagi T, Hirayama K, Ichinose A, Tanaka T, et al. Trypanocidal activity of extracts and compounds from the stem bark of Anogeissus leiocarpus and Terminalia avicennoides. See comment in PubMed Commons below Parasitol Res. 2008; 102: 697-703.
- Feyera T, Terefe G, Shibeshi W. Phytochemical Screening and in vitro Antitrypanosomal activity of the aerial parts of Artemisia abyssinica against Trypanosoma congolense Field Isolate. Ethiopian Pharmaceutical Journal 2011; 29: 137-142.
- Feyera T, Teref G, Shibeshi W. Evaluation of in vivo antitrypanosomal activity of crude extracts of Artemisia abyssinica against a Trypanosoma congolense isolate. BMC Complementary and Alternative Medicine 2014; 14: 117.
- Edwards S, Tadesse M, Hedberg I. Flora of Ethiopia and Erteria Canellacea to Euphorbiacea, The National Herbarium, Addis Ababa and Uppsala University 1995; 2: 286-290.
- Pascaline J, Charles M, Lukhoba C, George O. Phytochemical constituents of some medicinal plants used by the Nandis of South Nandi district, Kenya; Journal of Animal and Plant Sciences2011; 9: 1201- 1210.
- Runyoro DK, Ngassapa OD, Matee MI, Joseph CC, Moshi MJ. Medicinal plants used by Tanzanian traditional healers in the management of Candida infections. See comment in PubMed Commons below J Ethnopharmacol. 2006; 106: 158-165.
- Matu EN. Clutia abyssinica Jaub. & Spach. In: Schmelzer GH, Gurib-Fakim A, Editors. Prota11 (1): Medicinal plants/Plantes médicinales PROTA,Wageningen, Netherlands.2008.
- Njoroge GN, Bussmann RW. Traditional management of ear, nose and throat (ENT) diseases in Central Kenya. See comment in PubMed Commons below J Ethnobiol Ethnomed. 2006; 2: 54.
- Yineger H, Kelbessa E, Bekele T, Lulekal E. Ethnoveterinary medicinal plants at Bale Mountains National Park, Ethiopia. See comment in PubMed Commons below J Ethnopharmacol. 2007; 112: 55-70.
- Fullas F. Ethiopian medicinal plants in veterinary health care. Ethiopian e-journal for research and innovation. 2010; 2: 48-58.
- Murray M, Murray PK, McIntyre WI. An improved parasitological technique for the diagnosis of African trypanosomiasis. See comment in PubMed Commons below Trans R Soc Trop Med Hyg. 1977; 71: 325-326.
- Uilenberg G. Field guide for diagnosis, treatment and prevention of African animal trypansomosis. Food and agriculture organization of the United Nations Publications, Rome. 1998.
- ILAR [Institute for Laboratory Animal Research, National Research Council]. Humane endpoints for animals used in biomedical research and testing. ILAR. 2000; 41: 59-123.
- Lorke D. A new approach to practical acute toxicity testing. See comment in PubMed Commons below Arch Toxicol. 1983; 54: 275-287.
- Moti Y, Fikru R, Van Den Abbeele J, Büscher P, Van den Bossche P, Duchateau L, et al. Ghibe river basin in Ethiopia: present situation of trypanocidal drug resistance in Trypanosoma congolense using tests in mice and PCR-RFLP. See comment in PubMed Commons below Vet Parasitol. 2012; 189: 197-203.
- Herbert WJ, Lumsden WH. Trypanosoma brucei: a rapid "matching" method for estimating the host's parasitemia. See comment in PubMed Commons below Exp Parasitol. 1976; 40: 427-431.
- Wintrobe MM. and Landsberg JW. A standardized technique for the blood sedimentation test. American Journal of medical science. 1970; 102-104.
- Wernery U, Zachariah R, Mumford JA, Luckins T. Preliminary evaluation of diagnostic tests using horses experimentally infected with trypanosoma evansi. See comment in PubMed Commons below Vet J. 2001; 161: 287-300.
- Yabu Y, Yoshida A, Suzuki T, Nihei C, Kawai K, Minagawa N, et al. The efficacy of ascofuranone in a consecutive treatment on Trypanosoma brucei brucei in mice. See comment in PubMed Commons below Parasitol Int. 2003; 52: 155-164.
- Nok AJ. Azaanthraquinone inhibits respiration and in vitro growth of long slender bloodstream forms of Trypanosoma congolense. See comment in PubMed Commons below Cell Biochem Funct. 2002; 20: 205-212.
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. See comment in PubMed Commons below Phytochemistry. 2000; 55: 481-504.
- Taylor L. Plant based drugs and medicine. Raintee Nutrition Inc. 2000; 1-5.
- Nibret E, Wink M. Volatile components of four Ethiopian Artemisia species extracts and their in vitro antitrypanosomal and cytotoxic activities. See comment in PubMed Commons below Phytomedicine. 2010; 17: 369-374.
- Saeidnia S, Gohari AR, Uchiyama N, Ito M, Honda G, Kiuchi F, et al. Two new monoterpene glycosides and trypanocidal terpenoids from Dracocephalum kotschyi. See comment in PubMed Commons below Chem Pharm Bull (Tokyo). 2004; 52: 1249-1250.
- Abd E Hady FK, Hegazi AG. Egyptian propolis: 2. Chemical composition, antiviral and antimicrobial activities of East Nile Delta propolis. See comment in PubMed Commons below Z Naturforsch C. 2002; 57: 386-394.
- Pelttari E, Matikainen J, Elo H. Antimicrobial activity of the marine alkaloids haminol and pulo'upone and related compounds. See comment in PubMed Commons below Z Naturforsch C. 2002; 57: 548-552.
- Colegate SM, Molyneux RJ. Bioactive Natural Products Detection, Isolation, and Structural Determination. CRS press Inc. 1993; 423.
- Cos P, Hermans N, De BT, Apers S, Sindambiwe JB, Witvrouw M, et al. Antiviral activity of Rwandan medicinal plants against human immunodeficiency virus type-1 (HIV-1). See comment in PubMed Commons below Phytomedicine. 2002; 9: 62-68.
- Ogbadoyi E, Adamu Y, Omotosho R. Preliminary studies of the antitrypanosomal activity of Garcinia kola nut extract in mice infected with T. b. brucei. Journal of Medicine and Medical Sciences 2011; 2: 628-631.
- Mann A, Egwim EC, Banji B, Abdukadir N, Gbate M, Ekanem JT. Efficacy of Dissotis rotundifolia on Trypanosoma brucei brucei infection in rats. African Journal of Biochemistry Research. 2009; 3: 5-8.
- Williams S, Saha L, Singha UK, Chaudhuri M. Trypanosoma brucei. Differential requirement of membrane potential for import of proteins into mitochondria in two developmental stages. Experimental Parasitology 2005; 118: 420–433.
- Afewerk Y, Clausen PH, Abebe G, Tilahun G, Mehlitz D. Multiple-drug resistant Trypanosoma congolense populations in village cattle of Metekel district, north-west Ethiopia. See comment in PubMed Commons below Acta Trop. 2000; 76: 231-238.
- Assefa E, Abebe G. Drug-resistant Trypanosoma congolense in naturally infected donkeys in north Omo Zone, southern Ethiopia. See comment in PubMed Commons below Vet Parasitol. 2001; 99: 261-271.
- Delespaux V, Geysen D, Van den Bossche P, Geerts S. Molecular tools for the rapid detection of drug resistance in animal trypanosomes. See comment in PubMed Commons below Trends Parasitol. 2008; 24: 236-242.
- Miruk A, Hagos A, Yacob HT, Asnake F, Basu AK. Prevalence of bovine trypanosomosis and trypanocidal drug sensitivity studies on Trypanosoma congolense in Wolyta and Dawero zones of southern Ethiopia. See comment in PubMed Commons below Vet Parasitol. 2008; 152: 141-147.
- Losos GJ, Iked BO. Review of the pathology of domestic and laboratory animal caused by T. congolence, T. vivax, T. brucei, T. rhodesiense and T. gambiense. Veterinary Pathology. 1972; 9: 1-79.
- Maxie MG, Losos GJ. Release of Trypanosoma congolense from microcirculation of cattle by berenil. Veterinary Parasitology. 1977; 3: 277-281.
- Mbaya AW, Nwosu CO, Onyeyili PA. Toxicity and anti-trypanosomal effects of ethanolic extract of Butyrospermum paradoxum (Sapotaceae) stem bark in rats infected with Trypanosoma brucei and Trypanosoma congolense. See comment in PubMed Commons below J Ethnopharmacol. 2007; 111: 526-530.
- Albert M, Hussein K. The Mechanisms of anemia in trypanosomiasis: A Review, Anemia, Donald Silverberg, Editors. 2012: 978-953-51-0138-3.
- Ekanem JT, Kolawole OM, Abbah OC. Some biochemical and haematological effects of black seed (Nigella sativa) oil on Trypanosoma brucei infected rats. African Journal of Biochemistry Research. 2008; 2: 45 -50.
- Mpiana PT, Tshibangu DS, Shetonde OM, Ngbolua KN. In vitro antidrepanocytary actvity (anti-sickle cell anemia) of some congolese plants. See comment in PubMed Commons below Phytomedicine. 2007; 14: 192-195.
- Ogoti P, Magiri E, Auma J, Magoma G, Imbuga M, Murilla G. Evaluation of In vivo antitrypanosomal activity of selected medicinal plant extracts. Journal of Medicinal Plant Research. 2009; 3: 849-854.
- Inabo HI, Fathuddin MM. In vivo antitrypanosomal potentials of ethyl acetate leaf extracts of Punica granatum against T. b. brucei. Advances in Agricultural biotechnology. 2011; 1: 82-86.
- Umar IA, Ibrahim MA, Fari NA, Isah S, Balogun DA. In vitro and in vivo antitrypanosoma evansi activities of extracts from different parts of Khaya senegalensis. Journal of Cell and Animal Biology 2010; 4: 91-95.
- Abubakar A, Iliyasu B, Yusuf AB, Igweh AC, Onyekwelu NA, Shamaki BU, et al. Antitrypanosomal and haematological effects of selected Nigerian medicinal plants in Wistar rats. Biokemistri 2000; 17: 95-99.
- Adamu M, Nwosu CO, Igbokwe IO. Toxicity and Phytochemical Constituents of aqueous Extract of Ocimum gratissimum Leaf. Nigerian Veterinary Journal. 2008; 29: 48-57.
- Alli LA, Okochi VI, Adesoken AA. Antitrypanosomal activity and hematological effects of aquous extract of leaves of Morinda Lucida on Trypanosoma brucie brucie infected rats. Asian Journal of Pharmaceutical and Health Sciences. 2011; 1: 111-115.
- Holmes PH, Katunguka-Rwakishaya E, Bennison JJ, Wassink GJ, Parkins JJ. Impact of nutrition on the pathophysiology of bovine trypanosomiasis. See comment in PubMed Commons below Parasitology. 2000; 120 Suppl: S73-85.
- Yusuf AB, Umar IA, Musa UB, Nok AJ. Screening of Vernonia amygdalina and Hymenocardia acida extracts and 1,3-diaminopropane for their antitrypanosomal activities: in vitro model. Journal of Medicinal Plant Research. 2012; 6: 3573-3578.