
Research Article
Austin J Pharmacol Ther. 2022; 10(1).1162.
Investigation of Biomarkers in Sub-Acute Hepatorenal Toxicity against Bulk and Zinc Oxide Nanoparticles Exposed Mice
Monika Sudhakar Deore¹, Jasleen Kaur¹ and Saba Naqvi1,2*
1Department of Pharmacology and Toxicology, National Institute of Pharmaceutical Education and Research (NIPER-R), India
2Department of Regulatory Toxicology, National Institute of Pharmaceutical Education and Research (NIPER-R), India
*Corresponding author: Saba Naqvi, National Institute of Pharmaceutical Education and Research- Raebareli, Lucknow (UP) - 226002, India; Email: saba. naqvi@niperraebareli.edu.in; writetosaba@yahoo.com; ORCID: 0000-0001-6591-790X
Received: July 15, 2022; Accepted: August 10, 2022; Published: August 17, 2022
Abstract
Zinc oxide nanoparticles have been utilized and produced at a very large scale due to their wide range of applications. Nevertheless, its toxicity is one of the concerns addressed by various researchers. Widely used methods of measuring plasma zinc have poor sensitivity and impaired specificity. Currently, there is no specific biomarker for determination of excess zinc inside our body is explored well. Furthermore, it is vital to know the toxic effects of zinc oxide nanoparticles and their bulk counterparts at early exposure level on human health due to day to day increased use of zinc oxide nanoparticles in various applications. In current study we investigate the kidney and liver as the primary target organs for toxicity and their oxidative stress parameters. Twenty-four male mice were divided into three groups(n=8). Control (group I) served as vehicle control; group II:50mg/kg ZnO (bulk) and group III:ZnO NPs (50mg/kg nano). The mice were sacrificed after 14 days exposure, liver and kidney tissue toxicity biomarkers were performed. Our results demonstrate increased levels of GPx, metallothionein, GST, and decrease level of ceruloplasmin, GSH:GSSG ratio in bulk ZnO administered animals. The acute kidney toxicity was further confirmed by increased levels of their biomarkers i.e. Kim-1 and Clusterin. The levels of serum cytokines and caspase were also analyzed. Hence, this study investigated the comparative effect of early exposure of zinc oxide nanoparticles (nZnO) and its bulk form and we conclude that at 50mg/kg b.wt dose, ZnO NPs is comparatively safe to bulk ZnO.
Keywords: Zinc oxide nanoparticles; Bulk zinc oxide comparison; Clusterin, KIM-1; Ceruloplasmin; Metallothionein; GSH; GSSG ratio; Glutathione peroxidase
Introduction
Zinc oxide nanoparticles(ZnO NPs) are already known for a wide variety of applications because of their enhanced and exceptional properties over their bulk form. ZnO NPs are released into the aquatic system through domestic and industrial wastewater [1]. Researchers have investigated the toxicity of ZnO NPs over the years and confirmed the toxicity in both in-vitro and in-vivo depending on various parameters such as size, dose, and duration. The studies conducted so far have shown toxicity to most of the organs including the liver, kidney, brain, and spleen, etc. However, these studies were limited because of no much comparison with its bulk form and others. Also, no single study confirmed the detailed mechanisms and pathways involved in toxicity in addition to its investigating interlink of this pathology. Studies conducted so far showed the oxidative stress mechanism involved in ZnO NPs toxicity [2]. Thus the generated ROS further causes oxidative DNA damage, cytotoxicity, genotoxicity, etc [3]. ZnO NP is known to be more toxic than other metal oxide nanoparticles due to its ability to shed Zn2+ upon particle dissolution [3,4]. Analysis of the oxidative stress enzyme such as GPx and GST activity upon exposure to ZnO NPs is considered one of the parameters of their toxicity [5]. These are other oxidative stress biomarkers involved in redox cycling. Oxidative stress requires rapid detoxification before it impairs other cellular processes. Early biomarker assessment is essential to tackle any kind of toxicity [6-8]. ZnO NP is exposed to humans as well as animals mainly via dermal, inhalation, and oral routes [9].
Currently, there is a lack of satisfying biomarkers for the assessment of zinc status. However, plasma zinc measurement is the most widely and accepted biomarker of zinc status; but, it has poor sensitivity and impaired specificity. Zinc has been used as a nutritional supplement in various health conditions [10]. Nevertheless, an unusually high zinc intake might cause side effects such as fever, coughing, fatigue, stomach pain, and many other problems including cancer [11-13]. Therefore, the utility of reliable biomarkers in assessing zinc status is essential for the detection of potentially toxic intakes [8,10]. Excess zinc causes copper deficiency which is further affects iron metabolism, causes oxidative stress, weakens the immune system, and decreases the cognitive ability in Alzheimer’s disease [13,14].
Glutathione Peroxidase-1 (GPx-1) is an intracellular antioxidant enzyme function like catalase, it also detoxifies hydrogen peroxide generated by catabolism of superoxide anions to water to limit its harmful effects [15,16]. GPx-1 expression is unique in the regulation of oxidative stress as it contains selenocysteine and is involved during translation hence in the development and prevention of many common and complex diseases [17]. Also, it modulates cell responses such as apoptosis or inflammation to drug toxicity, ischemiareperfusion injury, etc [16].
The cytosolic Glutathione S-Transferase (GST) is an important phase II biotransformation enzyme that catalyzes a nucleophilic attack by glutathione sulfur atom [18]. GST enzymes are involved in the detoxification reactions by conjugation to glutathione. GSTs have served as ideal early biomarkers of organ damage applicable to both human and animal models. Also, they are regiospecifically located in the liver and kidney [19]. GSTs are phase 2 conjugation enzymes protecting the cells from oxidative stress [20]. GST plays a role in preventing oxidative damage by conjugating breakdown products of lipid peroxides to GSH and thus generating less toxic and more hydrophilic molecules [15]. Monitoring of hepatic function is done by liver enzymes according to the genetic polymorphism in early-phase treatment. So, GST is known to be a sensitive biomarker compared to liver enzymes. The specificity of GSTs is due to their large hepatic distribution, high cytosolic concentration, and short plasma half-life [20]. GST is a potential biomarker that can give early warning in nZnO toxicity [21]. Hence, the GSH: GSSG ratio is also a potential biomarker of oxidative stress shows the redox status of the cells. The imbalance in this ratio is indicative of oxidative stress [22].
Ceruloplasmin, a ferroxidase is an alpha-globulin that is involved in both copper and iron homeostasis. It plays an important role in iron transport by oxidizing ferrous iron to the ferric form thus promoting the loading onto transferrin. Oxidizing ferrous to ferric iron also has an antioxidant function [23]. It was reported that high zinc level blocks intestinal absorption of copper [24]. Ceruloplasmin (Cp) is a known biomarker assessed in copper metabolism [25]. In current study, we have evaluated the ceruloplasmin level in ZnO NPs exposed groups, ceruloplasmin catalyzes redox reactions. The lower level indicates the cupper deficiency and also indicates the hypoproteinemic state [26]. The low copper level is also indicated by anemia, and neutropenia [27]. Copper is required for physiological processes such as hemoglobin synthesis, iron oxidation, antioxidant defense peptide amidation, etc. [28].
Metallothionein (MTs) are cysteine-rich low molecular weight proteins that play a role in metal homeostasis [29]. Their levels are known to be overexpressed in response to high metal concentrations. Metal from the GSH–metal complexes involved in metal metabolism is further transferred to MT apoproteins. MT has thus shown its usefulness in environmental monitoring even in complex environments where interference of other xenobiotics can be found. [15]. It detoxifies the heavy metal from the body upon exposure to heavy metal [30]. Metallothionein has also been investigated along with ceruloplasmin levels in zinc exposed rats [31].
Previous studies on renal toxicity showed the mechanisms such as changes in liver enzymes, oxidative stress, inflammation, DNA damage and apoptosis etc. [32]. Among many methods used for the assessment of kidney function, the most widely used method is the measurement of serum creatinine, glomerular function rate (GFR) and urea. But, measuring GFR is time consuming and tedious method [33]. Moreover, serum creatinine level and glomerular filtration rate are detectable when kidney function becomes half. Although Acute Kidney Injury (AKI) progresses towards Chronic Kidney Disease (CKD) as a long-term consequence, the exact pathogenesis of AKI to CKD is largely unknown [34]. Delay in CKD diagnosis cause deterioration of nephron function which furthermore causes endstage renal disease and at that point patients require dialysis or kidney transplant [35].
KIM-1 is one type I transmembrane protein barely expressed in normal kidneys. Increased KIM-1 level is a novel sensitive biomarker for evaluating early kidney damage as compared to traditional biomarkers such as creatinine. Thus, this suggests acute kidney injury particularly in proximal tubular epithelial cells [36-38]. It may play an important role in early tubular epithelial cell damage by modulating damage and repair mechanisms [39]. Food and Drug Administration (FDA) and the European Medicines Agency (EMEA) confirmed KIM-1 as a highly sensitive and specific biomarker [39]. Clusterin is also a newly identified glycoprotein biomarker that shows association with tubulointerstitial renal lesions. Similar to KIM-1 it also serves as the early biomarker of tubular injury [39].
Our study aimed at investigating the early biomarker in target organ like kidney and liver of bulk and nano ZnO exposed mice. This would help in comparing the early exposure toxicity of bulk zinc oxide and ZnO NPs and further helps in designing its prevention and treatment methodology at an early stage.
Materials and Methods
Drugs and chemicals
Zinc oxide-nanopowder and ZnO were purchased from Sigma Aldrich Co., St Louis, Missouri, USA. Enzyme-linked immunosorbent assay (ELISA) kits for analysis of KIM-1 (Cat # ELK-1165), Clusterin (Cat # ELK-1310) were procured from ELK Biotechnology (Hubei, P.R.C).All other chemicals used in the study were of analytical grade.
Animals
Twenty-four male albino mice (weighing: 25-30 g) were procured from CSIR-Central Drug Research Institute, Lucknow, India. The mice were kept in the animal house facility of the National Institute of Pharmaceutical Education and Research (NIPER), Raebareli which is approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). The animal experimental protocols were approved by the Institutional Animal Ethics Committee (IAEC), NIPER-Raebareli. Standard laboratory animal feed (purchased from altromin, Im Seelenkamp Lage, Germany) and water (Aquapure) were provided ad libitum. The metal contents of the animal feed (in mg/kg) were (Al: 79.37, Cl: 3,484.07, Fe: 192.51, F: 2.80, I: 1.66, Co: 0.34, Cu: 12.81, Mg: 95.06, Mo: 1.10, S: 1,141.22, Se: 0.25, Zn: 95.18). Animals were acclimatized to the experimental conditions for one week before the start of the experiment. All the animal experimental procedures were performed as per the guidelines specified by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Characterization of ZnO NPs
The size and morphology of ZnO NPs were analyzed by transmission electron microscopy (TEM, JEM-200CX) at the CSIRIITR, Lucknow, India. One drop of the ZnO NPs was taken put onto the carbon-coated copper grid and left for 10 min. The excess amount of sample was removed carefully and then negative staining was done using 2% Phosphotungstic Acid (PTA). The samples were air-dried for 15 min and viewed under the TEM.
The hydrodynamic size and surface charge of the nanoparticles were determined using the Malvern Zetasizer Nano-ZS instrument at the National Institute of Pharmaceutical Education and Research (NIPER), Raebareli, Lucknow, India.
Dose Preparation
The ZnO NPs suspension was made using deionized water to minimize reactive oxygen species generation during sonication. Then, the suspension was sonicated for 20 min in a bath sonicator. Bulk ZnO powder was also mixed in deionized water and vortexed before administration to the animals.
Experimental Design
Twenty-four (24) male mice were divided into three groups of 8 each and were administered with normal water, Zinc oxide bulk, and nanoparticles (50 mg/kg, orally through gavage) for 14 days.
Group 1: Normal control
Group 2: Zinc oxide nanoparticles (50mg/kg, p.o.)
Group 3: Bulk Zinc oxide (50mg/kg, p.o.)
Daily body weight, food intake, and water intake were recorded. After 14 days of treatment, blood was collected from retro orbital plexus of animals and then sacrificed.
Functional Observational Battery (FOB): Functional observational battery (FOB) assessments were recorded for 6 animals/groups after completion of exposure. Testing was performed by trained personnel. The FOB was performed in a separate animal room. Animals were observed for the parameters listed in Table 1, which are based on previously developed protocols [40-43].
Home cage observations
Handling observations
Open field observations (performed over a 2-min period)
Sensory observations
Posture
Ease of removal from cage
Mobility
Approach response
Biting
Ease of handling animal in hand
Gait
Touch response
Convulsions/tremors
Salivation
Rearing
Tail pinch response
Feces consistency
Lacrimation
Arousal
Pupil response
Piloerection
Convulsions/tremors
Eyeblink response
Fur appearance
Urination/defecation
Mucous membranes/eye/skin color
Bizarre/stereotypic behavior
Table 1: Functional Observational (FOB) parameters evaluated in rats.
Blood and Tissue Sample Collection: The mice were sacrificed after 14 days of exposure by cervical dislocation and blood was collected by retroorbital puncture and transferred in tubes. Whole blood was centrifuged for 15 min at 2500 rpm to collect serum that was used for the estimation of ELIZA parameters. The liver and kidney were excised from the body and rinsed in ice-cold saline. Organ weight was recorded relative to body weight after wiping with blotting paper. The tissue homogenate of the liver was prepared as per the respective protocols by homogenizing at 2000g and centrifugation at 10,000g for 15min, and the supernatant was collected for estimation of various biochemical parameters.
Biochemical Parameters
Determination of Proteins: The protein concentration in tissue was determined by Folin’s Ciocalteau phenol reagent (Lowry et al., 1951). The Reagent C which is the alkaline copper reagent [Reagent C = 50ml = 50mL of Reagent A (2% sodium carbonate in 0.1 N NaOH) + 1mL of Reagent B (0.5% copper sulphate in 1% sodium potassium tartrate)] of 1ml was added to the 200μL of tissue homogenates and incubated at the room temperature for 10 min. After adding Reagent C, the Folin’s Ciocalteau phenol reagent (Folin’s Ciocalteau phenol reagent with water at the ratio of 1:1) of 100 μL was added to the mixture and incubated in dark at RT for 30 min. Bovine serum albumin (BSA) was used as the standard in the range of 10–640 μg which was also treated in the same manner as described above. The blue color was developed and absorbance was determined at 660nm [44].
Determination of Rreduced Glutathione Assay (GSH): The reduced glutathione (GSH) was proceeded by using the fluorometric method. The tissue homogenate was prepared by weighing 250mg of tissue in 3.75ml of phosphate EDTA buffer and 1 mL of 25% HPO3 (for precipitating proteins). The mixture was centrifuged for 30min. at 10,000g at 4°C and the supernatant was separated. For GSH, 0.5ml of supernatant was dissolved in 4.5 mL of phosphate EDTA buffer, pH 8.0. The mixture was vortexed. Take 100μl of the above mixture and mix with 1.8ml phosphate EDTA buffer and 100μl of O-Phthalaldehyde solution (prepared in absolute methanol). The resultant mixture was incubated at room temperature for 15min. The standards were prepared from 10μm GSH solution in the range of (0.2-6.4μm) and performed in similar manners as the sample. The fluorescence was measured at the emission of 420nm and excitation of 350nm [45].
Determination of Oxidized Glutathione Assay (GSSG): The oxidized glutathione was preceded by using the fluorometric method. The tissue homogenization process involved in this assay was similar to that of GSH. The resultant supernatant of 0.5ml was added to 200μl of N-ethylmaleimide (NEM) and incubated for 25 min. at room temperature. Take 100μl of the above solution was taken and mixed with 1.8ml phosphate EDTA buffer and 100 μl of o-Phthal-aldehyde solution (prepared in absolute methanol). The resultant mixture was incubated at room temperature for 15min. The standards were prepared from 10μm GSH solution in the range of (0.2-6.4μm) and performed in similar manners as the sample. The fluorescence was measured at the emission of 420nm and excitation of 350nm [45].
Determination of Glutathione Peroxide (GPx) Activity: The assay was performed according to the slight modifications of the reported method. The 20% tissue homogenization was prepared with phosphate buffer (PB) and 0.1ml was taken and mixed with 0.1ml of 5mM GSH, 0.1ml of 25mM sodium azide, 0.1ml of 1.25mM H2O2, and makeup to 2.5ml Phosphate Buffer(PB). The resultant mixture was incubated for 37°C for 10min. The reaction was terminated by adding 2ml of 1.65% metaphosphoric acid and centrifuged at 1500rpm for 10min. The 2ml supernatant was taken and mixed with 2ml of 0.4M disodium hydrogen phosphate and 1ml of 1mM DTNB. The resultant mixture was incubated for 10min. at 37°C. The absorbance was recorded at 420nm. The blank was prepared similarly as mentioned above instead of 0.1ml supernatant we added 0.1PB [46].
GST Activity Assay: GSTs activity was performed as per the Seyyedi et al 2005. Briefly, reduced glutathione (GSH) and 100 mM 1-chloro-2, 4-dinitrobenzene (CDNB) were taken as substrates and the reaction was performed at 25°C. Blank was performed similarly by taking phosphate buffer. The reaction kinetics was measured at 340nm every1 minute, for 15 min.
Ceruloplasmin Activity: The ceruloplasmin activity was measured in the serum according to the protocol described by Schosinsky et al. 1974. To evaluate the ceruloplasmin activity serum samples were mixed with the 0.1 M acetate buffer (pH 5.0) and incubated at 37oC for 5 min in a water bath. Following, O-dianisidine dihydrochloride (7.88 mM) was added to the sample, after 15 min incubation the reaction was stopped using sulphuric acid, and the developed red color was measured at 540 nm (Schosinsky et al 1974).
Metallothionein Determination: The metallothionein (MTs) content was measured by a modified method [47]. Tissue samples were homogenized in Tris buffer solution (30 mM Tris, 1.3 mM β-mercaptoethanol, 0.5 mM phenylmethylsulfonyl fluoride, 0.4 M sucrose). The homogenate was centrifuged at 21 000g for 60 min at 4°C. The supernatant was removed and treated with ethanol/ chloroform solution to obtain the metalloproteins. The MTs reaction mixture consisted of 0.6 mM DTNB (5,5'-dithiobis(2-nitrobenzoic acid)), 2 M NaCl, and 0.2 M phosphate buffer, pH 7.8. After 20 min, the MTs content was quantified spectrophotometrically at 412 nm, using glutathione as the standard. Results were expressed as units per min per mg protein [48].
Caspase-3 and Cytokine Analysis: The secretory levels of cytokines iNOS, IL-6, IL-1β, TNF-a, and caspase-3 in the mice serum were quantitatively determined using ELISA kits (ELK Biotechnology, BOSTER, Wuhan, China) according to the manufacturer’s instructions (Catalog no.TNF-a: :ELK1395, IL-1β : ELK-1271, NOS2:ELK-1504 and CASP3:ELK-1529. Clusterin, KIM-1 and cystatin-3 levels were also estimated in mice kidney using ELIZA kits as per the instructions provided by the manufacturer.
Statistical Analysis
All data are presented as mean ± SEM for each group. Statistical differences between the groups were determined by one-way analysis of variance followed by multiple comparisons with Tukey’s test using GraphPad Prism statistical software (version 6). The data were considered statistically significant at P < .05.
Results
Characterization of Zinc Oxide Nanoparticles by Transmission Electron Microscopy (TEM), Dynamic Light Scattering, and Zeta Potential Measurement
ZnO NPs were characterized for determining the particle size, size distribution, and zeta potential using a Zetasizer as shown in (Figure 1). The size and shape of the zinc oxide nanoparticles were analyzed by Transmission Electron Microscopy (TEM). Figure 1A depicts the spherical shape and monodispersed nature of zinc oxide nanoparticles, with an average particle size of around 60nm. The average hydrodynamic diameter of ZnO NPs was around 60–70 nm (Figure 1C). Nanoparticle exhibited a negative charge, that is, -21.2, which was confirmed by the zeta potential as shown in figure (Figure 1B).
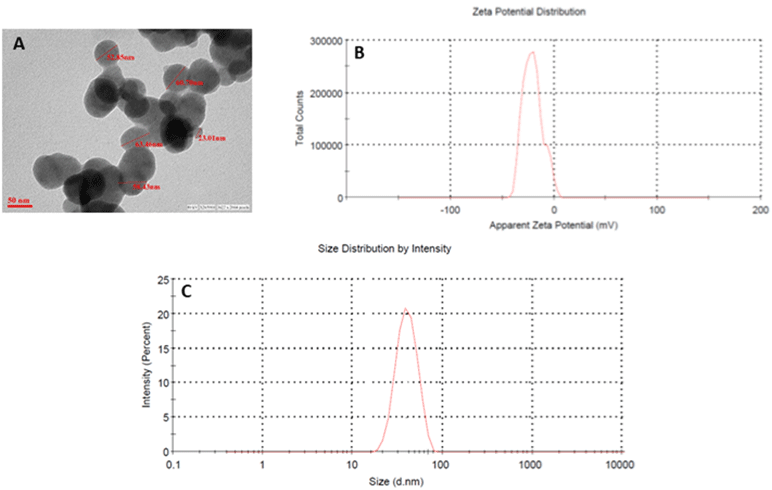
Figure 1: Characterization of Zinc oxide nanoparticles. (A) Transmission electron microscopy; (B) zeta potential (C) the hydrodynamic size of NPs determined by
dynamic light scattering (DLS) studies.
Body weight, food, and water intake were recorded daily for 14 days. Bulk zinc oxide exposed animals showed a significant reduction in body weight which was in coherence with the significant reduction with feed intake. Whereas, the nano ZnO group did not show such changes as compared to control groups (Figure 2A & B). Moreover, Bulk ZnO groups also showed decreased water intake as compared to nano ZnO group animals (Figure 2C).
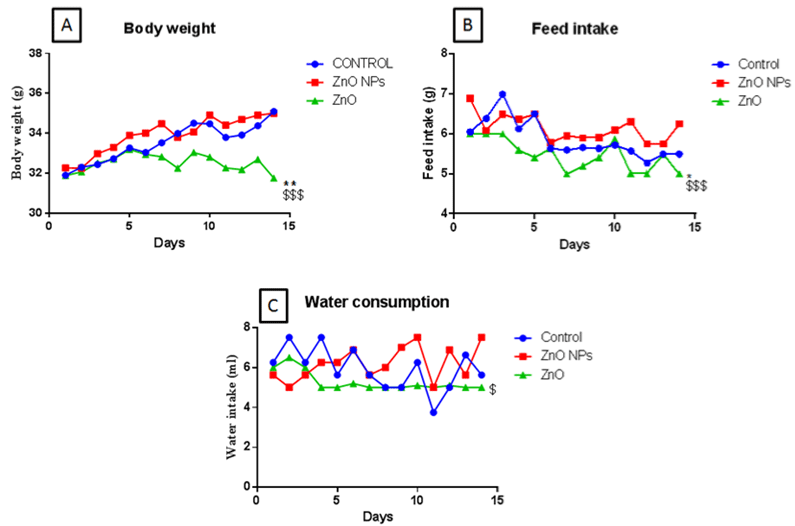
Figure 2: Body weight (A), feed intake (B), and water consumption (C) of mice following gavage administration off nano and bulk ZnO for 15 days (50 mg/kg). All the
values are expressed as mean±SEM (n=8). The statistical significance was considered at p < 0.05 and statistical analysis was performed using one-way ANOVA
followed by Turkey’s test. *p<0.05, **p<0.01, Vs Control ; $$p <0.01 VsZnO NPs.
Functional Observational Battery (FOB) and Motor Activity
We have not observed any effect during the FOB (data not presented) on the home cage, handling, open field, and sensory parameters both in bulk ZnO and nano ZnO. Ambulatory and total motor activity (data not presented) were unaffected in all groups.
Biochemical Parameters in Kidney
There was a significant increase in GPx and metallothionein and a slight increase in GST of bulk ZnO exposed animals and no significant changes were observed in nano ZnO exposed animals (Figure 3A, D & B resp). Furthermore, there was a significant decrease in GSH: GSSG ratio of bulk ZnO exposed animals (Figure 3C) and no such changes were observed in nano ZnO exposed animals.
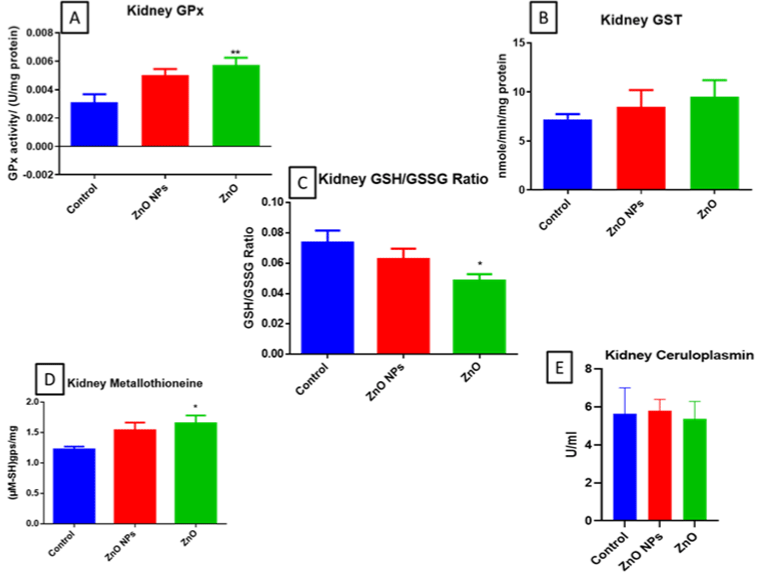
Figure 3: GPx (A), GST (B), GSH: GSSG ratio (C), Mettallothinein (D), and Ceruloplasmin (E) levels in mice kidney. All the values are expressed as mean±SEM
(n=6). The statistical significance was considered at p < 0.05 and statistical analysis was performed using one-way ANOVA followed by Turkey’s test. *p< 0.05,
**p< 0.01, Vs Control. GPx: Glutathione peroxidase; GST: Glutathione S-transferase.
Biochemical Parameters in Liver
Bulk ZnO exposed animals showed GPx and GST activity and a significant increase in metallothionein (Figure 4A, B & D). No such changes were observed in the nano ZnO group except an increase in metallothionein (Figure 4D). Moreover, there was a decrease in GSH:GSSG ratio, and ceruloplasmin levels were observed in bulk ZnO exposed groups (Figure 4C & E resp).
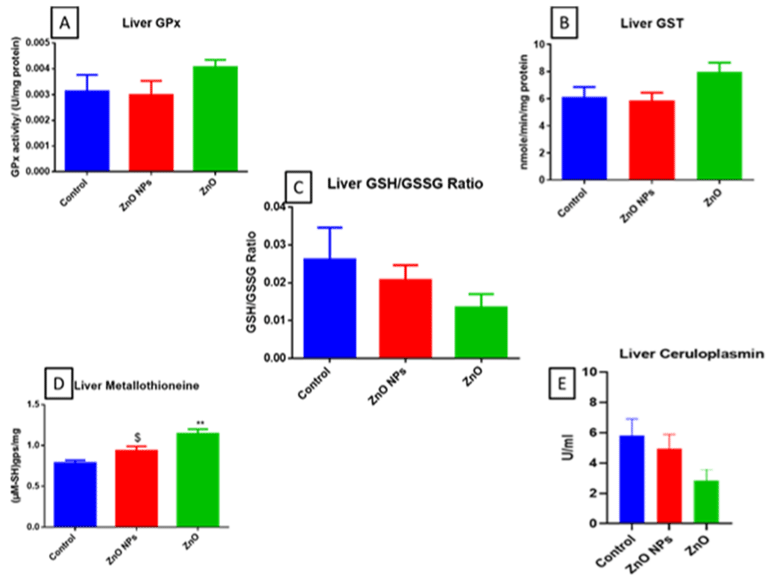
Figure 4: GPx (A), GST (B), GSH: GSSG ratio (C), Mettallothinein (D), and Ceruloplasmin (E) levels in mice liver. All the values are expressed as mean±SEM
(n=6). The statistical significance was considered at p < 0.05 and statistical analysis was performed using one-way ANOVA followed by Turkey’s test. *p< 0.05,
**p< 0.01, Vs Control; $p , 0.05 Vs ZnO NPs.
Kidney Specific Biomarkers
The mouse serum levels of KIM-1 and Clusterin were significantly (**p< 0.01) increased in bulk ZnO exposed groups as compared to control. Whereas no significant changes in nano ZnO exposed animals were found (Figure 5A & B). However, There was no significant change in cystatin-3 levels.
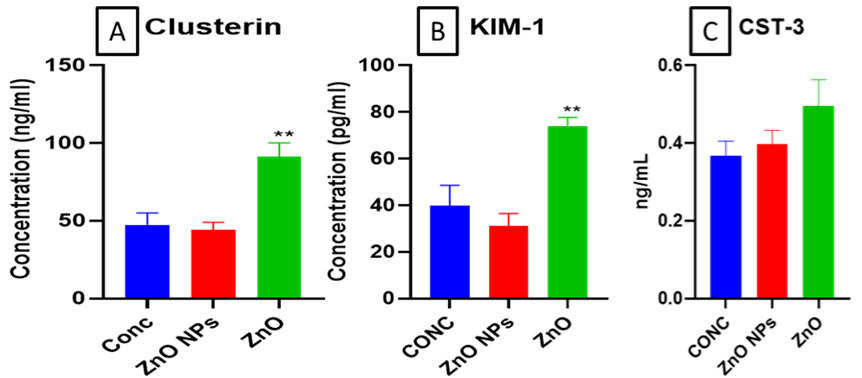
Figure 5: Levels of (A) CLU (Clusterin), (B) Kidney Injury Molecule-1 (KIM-1) and (C) Cystatin-3 in mouse serum determined using ELIZA. All the values are
expressed as mean±SEM (n=6). GPx (A), GST (B), GSH: GSSG ratio (C), Mettallothinein (D), and Ceruloplasmin (E) levels in mice kidney. All the values are
expressed as mean±SEM (n=6). The statistical significance was considered at p < 0.05 and statistical analysis was performed using one-way ANOVA followed by
Turkey’s test. **p< 0.01, Vs Control.
Organ Weights
There were no significant differences in organ weights of both bulk and nano ZnO exposed groups were reported (Fig. 6A & B). However, there was a significant decrease (*p< 0.05) in relative kidney and liver weights of bulk ZnO exposed animals were observed. However, no such changes were observed in nano ZnO exposed animals (Figure 6C & D resp).
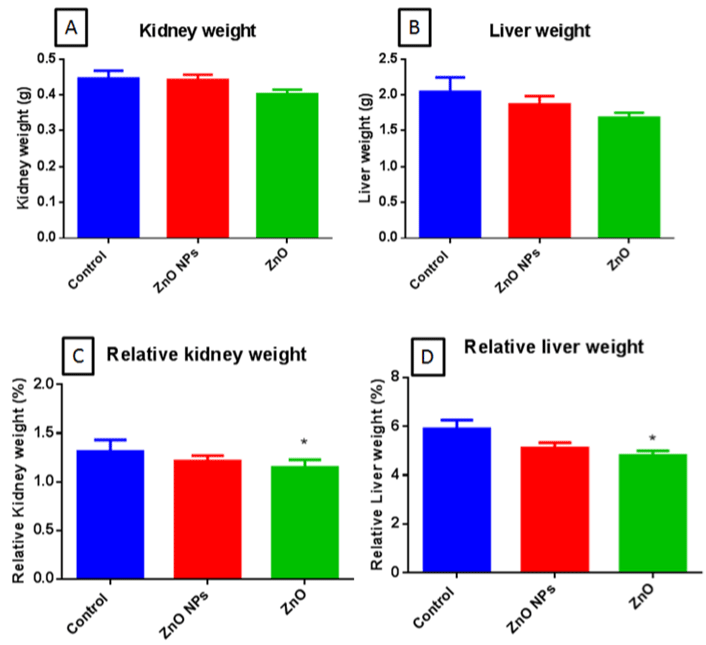
Figure 6: Kidney weights (A), Liver weight (B), relative kidney weight (C), and relative liver weight (D) in mice. All the values are expressed as mean±SEM (n=6).
The statistical significance was considered at p < 0.05 and statistical analysis was performed using one-way ANOVA followed by Turkey’s test. *p< 0.05, Vs Control.
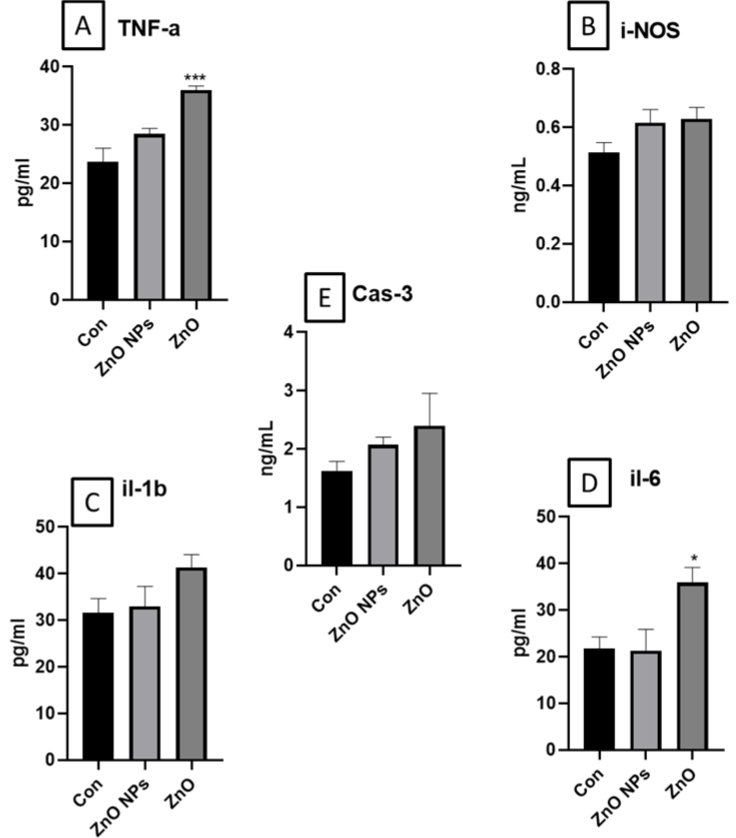
Figure 7: Illustrates the cytokines A. TNF-alpha; B. IL-1 beta; C. IL-6; D. i-NOS and E. caspase-3 levels in mouse serum. All data was represented as Mean±SEM
(n=4). Statistical analysis was carried out by using one-way ANOVA followed by Turkey’s multiple comparison post-hoc test. The statistical significance was
considered at p<0.05. *p(0.05, **p<0.01, ***p<0.001vs control.
Measurement for Serum Cytokines And Caspase-3
We have found in significant increase in TNF-a and IL-6 in ZnO exposed animals. Further, a slight and insignificant increase of i-NOS, caspase-3 and IL-1 β was observed in ZnO group. Whereas ZnO NP exposed group did not show any significant alterations in these levels.
Discussion
We investigated that ZnO NPs of size range of 60-70nm having zeta potential of approximately -21.1. Analysis of organ weight is an important endpoint for the identification of potentially harmful chemicals in toxicity studies. A difference in organ weight is accompanied by body weight differences which make the interpretation difficult. Therefore, relative organ weight is considered more accurate to find target organ toxicity [49]. Our results showed decreased bodyweight, food intake and water consumption in ZnO treated groups in comparison to control. In contrast, the nano ZnO treatment group showed a slight increase in body weight food intake, and water consumption.
Robert J. Cousins et.al. have shown ceruloplasmin and metallothionein induction by zinc. In our study, we found significantly increased metallothionein expression in both liver and kidney of bulk ZnO exposed groups [31]. Whereas, increased ceruloplasmin was observed in the liver and kidney ZnO NP group in comparison with the bulk ZnO group. Ceruloplasmin is synthesized in the liver & secreted in plasma and its concentration decreases in liver diseases. There are various mechanisms involved where the ceruloplasmin synthesizing capabilities of the liver decrease. One of the mechanisms for decreasing ceruloplasmin is zinc toxicity which induces copper deficiency. Ceruloplasmin functions as an antioxidant due to its ferroxidase I activity and thus prevents lipid peroxidation, scavenging superoxide & sequester-free copper ions [23,50]. An earlier study reports the association of changes in levels of the acute-phase proteins with lead-induced modifications of the immune system. Data from these studies also suggest that adequate dietary intake of the trace metals Fe, Zn, Se, and Cu is necessary as they reduce lead toxicity. They reported the positive correlations between the lead toxicity parameters and caeruloplasmin levels [51]. Overall earlier studies also concluded that lead exposure results in disorder of copper homeostasis [52].
Mitochondrial oxidative phosphorylation generates free radicals or reactive oxygen species (ROS) which causes oxidative damage by metabolites of pollutants [53]. GPx detoxifies ROS and hydroperoxides by oxidation of GSH to GSSG. GSSG is then reduced to GSH by GR which is recycled by the pentose phosphate pathway. GSSG accumulates and it is translocated outside the cell by specific transporters when GSSG generation is higher [21]. Hence, The GSH/GSSG ratio reflects the accumulation of GSSG, this is a more reliable indicator of cellular redox status [54]. Overall, cellular GSH is oxidized to glutathione disulfide (GSSG) to protect cells from the damage caused by ROS [55]. We have observed a significant decrease in GSH: GSSG ratio in kidney and liver of bulk but the nZnO treated groups showed a slight decrease as compared to the bulk ZnO group.
Excess GPx may also have deleterious effects and thus proper balance is required [16]. An earlier study has shown a decrease GPx and increased GST activity in response to exposure to a toxicant and was used as a biomarker of toxicity [15]. A decrease in GPx promotes ROS and oxidative stress which furthermore affects the structural and functional integrity of cell and organelle membranes [15]. Excess GPx has deleterious effects on cells and is the result of reductive stress [16]. Our study showed a significant increase in GPx activity in ZnO treated group.
As per the earlier studies, GST levels were not found to be levated even if the liver enzymes were elevated. There have been limited studies on GSTs and drug toxicity. Here, we are mainly focusing on investigating the impact of GSTs as an early biomarker in bulk and nano ZnO exposed liver and kidney [20]. Increased GST activity was suggested to neutralize the toxicants and thus to resist the toxicity of the pollutant (mainly metals) [15]. Our study showed increased GST activity in ZnO treated group. GST a multicomponent enzyme involved in the detoxification of many xenobiotics protects tissue from oxidative stress [21,56]. Upregulated Phase II enzymes have been used as biomarkers of exposure to chemicals [18].
Earlier studies also showed the Kim-1 overexpression in a variety of acute and chronic renal injuries in both preclinical and human kidney diseases caused by exposure to a variety of substances [57- 60]. In addition to upregulated urine KIM-1 also serve as a blood biomarker of kidney injury. It has been known that KIM-1 can detect renal tubular damage earlier than histological examination [39]. Only clusterin is secondary to KIM-1. Moreover, clusterin is considered as a pan biomarker as it detects the damage to glomeruli, tubules, and also collecting duct. Whereas KIM-1 detects damage associated with the proximal tubule. In this study, we have observed an increased level of both KIM-1 and clusterin which indicated the damage to the whole kidney. Further, we have measured the levels of cystatin-3 in kidney samples which is a biomarker of kidney function. As alteration in kidney function occurs in chronic or in late stages and thus the cystatin-3 levels. We also did not fing any significant change in the levels of cystatin-3.
Earlier studies have shown the normal tissue morphology when clusterin comes in tubular injury location and is thus used as an early biomarker of kidney damage.
Metallothionine along with protection against metal toxicity also involved in zinc and copper regulation. Also, they are primarily synthesized by the liver and kidney and localized in the cytoplasm. Metallothionein levels increase in oxidative stress to prevent cells from cytotoxicity and DNA damage. Metallothionein binds with nearly seven zinc atoms. Metallothionein binds with zinc and carriers it to different parts of the body. It is important in the brain where zinc signaling has a prominent role in its function. Also, zinc is a key element for the binding and activation of transcription factors. Levels MT was considerably elevated in earlier studies in response to metals. Physiologically MTs protect cells against the toxic effects of free oxygen radicals. In this way, MT scavenges oxyradical species and inactivates metals and protects the cells [15]. In this study, we observed a significant increase in metallothionein levels of both kidney and liver tissue of bulk ZnO exposed animals.
Kidney and liver weight of bulk ZnO treated groups were decreased vary slightly as compared to control. However, relative organ weights were significantly decreased in bulk ZnO treated groups as compared to control.
We have determined the levels of caspase 3 to determine whether the toxicity involves in a caspase dependent or independent pathway in response to acute insults. Cytokines show robust modulation in proximal events of inflammation, immune response, and repair. We have found insignificant increase in iNOS, caspase-3 and IL-1β levels in ZnO exposed mice whereas TNF-a and IL-6 levels were significantly increased. However, there was no change in levels of cytokines and caspase-3 in ZnO nanoparticle group. Earlier studies have shown the significant increase in two pro-inflammatory cytokines, TNF-a and IL-6 levels in mice orally treated with 10 mg/ kg/day of PU-NPs. Overall, increased cytokine expression are indicative of toxicity. Various studies evalauates the cytokines level as exploratory biomarker [61].
Conclusion
Bulk ZnO exposed mice showed significantly decreased in bodyweight, feed-water intake, whereas the nano ZnO exposed groups did not show such changes. GPx, metallothionein, and GST level were also high in both liver and kidney of bulk ZnO groups as compared to control. Whereas ceruloplasmin and GSH/GSSG ratio was decreased in both kidney and liver of bulk ZnO exposed animals. However, the decreased ceruloplasmin was not significant. Both organ toxicity to the kidney and liver was observed by decreased relative organ weight ratio upon exposure to bulk ZnO in comparison to its nano form. Altogether, excess zinc and its toxicity are mainly shown by increased metallothionein, GPx, and decrease GSH/GSSG ratio was observed in bulk ZnO as compared to its nano form. Similarly, increased TNF and IL-6 levels in bulk ZnO group indicative of acute toxicity. Overall, we observed that on evaluation of comparative effect of early exposure of zinc oxide nanoparticles (nZnO) and its bulk form in two major organ i.e. kidney and liver thus, we conclude that at 50mg/kg b.wt dose, ZnO NPs is comparatively safe to bulk ZnO.
Acknowledgment
Authors acknowledge the Department of Pharmaceuticals (DoP), Ministry of Chemicals and Fertilizers, Government of India, for their support. NIPER, Raebareli communication number for this manuscript is NIPER-R/Communication/285.
Author Contribution
MSD: literature search, animal dosing, biochemical assays, and illustration of the Figs, data calculation, statistical analysis; draft manuscripts; SN: conceptualized, overall supervision, manuscript review and finalization.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
The animals were given humane care as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India, and prior permission was taken from the Institutional Animal Ethics Committee of the National Institute of Pharmaceutical Education and Research, Raebareli.
References
- Beegam A, Prasad P, Jose J, Oliveira M, Costa FG, et al. Environmental fate of zinc oxide nanoparticles: risks and benefits. Toxicology-New Aspects to This Scientific Conundrum. 2016.
- Deore MS, S K, Naqvi S, Kumar A, Flora SJS. Alpha-Lipoic Acid Protects Co-Exposure to Lead and Zinc Oxide Nanoparticles Induced Neuro, Immuno and Male Reproductive Toxicity in Rats. Frontiers in Pharmacology. 2021; 12.
- Vimercati L, Cavone D, Caputi A, Maria LD, Tria M, Prato E, et al. Nanoparticles: An Experimental Study of Zinc Nanoparticles Toxicity on Marine Crustaceans. General Overview on the Health Implications in Humans. Frontiers in Public Health. 2020; 8.
- Xia T, Kovochich M, Liong M, Mädler L, Gilbert B, Shi H, et al. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS nano. 2008; 2: 2121-2134.
- Soto ME, Soria-Castro E, Lans VG, Ontiveros EM, Mejía BIH, Hernandez HJM, et al. Analysis of Oxidative Stress Enzymes and Structural and Functional Proteins on Human Aortic Tissue from Different Aortopathies. Oxidative Medicine and Cellular Longevity. 2014; 2014: 1-13.
- Sawyer DB, Siwik DA, Xiao L, Pimentel DR, Singh K, Colucci WS. Role of oxidative stress in myocardial hypertrophy and failure. Journal of molecular and cellular cardiology. 2002; 34: 379-388.
- Rush C, Nyara M, Moxon JV, Trollope A, Cullen B, Golledge J. Whole genome expression analysis within the angiotensin II-apolipoprotein E deficient mouse model of abdominal aortic aneurysm. BMC Genomics. 2008; 10: 298-298.
- Naqvi SF, SJS Flora. Nanomaterial's toxicity and its regulation strategies. Journal of Environmental Biology. 2020; 41.
- Dogan-Topal B, Uslu B, Ozkan SA. 2018. Chapter 14 - Detection of DNA damage induced by nanomaterials In Nanoscale Fabrication, Optimization, Scale-Up and Biological Aspects of Pharmaceutical Nanotechnology, ed. AM Grumezescu. William Andrew Publishing. 547-77.
- Hambidge M. Biomarkers of trace mineral intake and status. The Journal of nutrition. 2003; 133: 948S-955S.
- Parveen N, Ansari MO, Ahmad MF, Jameel S, Shadab G. Zinc: An element of extensive medical importance. Current Medicine Research and Practice. 2017; 7: 90-98.
- Khairnar P, Handa M, Shukla R. Nanocrystals: An Approachable Delivery System for Anticancer Therapeutics. Curr Drug Metab. 2022.
- Singh A, Ujjwal RR, Naqvi S, Verma RK, Tiwari S, Kesharwani P, et al. Formulation development of tocopherol polyethylene glycol nanoengineered polyamidoamine dendrimer for neuroprotection and treatment of Alzheimer disease. J Drug Target. 2022; 30: 777-91.
- Osredkar J, Sustar N. Copper and zinc, biological role and significance of copper/zinc imbalance. J Clinic Toxicol S. 2011; 3: 0495.
- Carvalho CDS, Bernusso VA, Araújo HSSD, Espíndola ELG, Fernandes MN. Biomarker responses as indication of contaminant effects in Oreochromis niloticus. Chemosphere. 2012; 89: 60-69.
- Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxidants & redox signaling. 2011; 15: 1957-1997.
- Kondaparthi P, Deore M, Naqvi S, Flora SJS. Dose-dependent hepatic toxicity and oxidative stress on exposure to nano and bulk selenium in mice. Environmental Science and Pollution Research. 2021; 28: 53034-53044.
- Coppock RW, Dziwenka MM. 2014. Biomarkers of petroleum products toxicity In Biomarkers in Toxicology, ed. RC Gupta, Boston: Academic Press. 2014. Chapter 37. pp. 647-54.
- Kilty C, Doyle S, Hassett B, Manning F. Glutathione S-transferases as biomarkers of organ damage: applications of rodent and canine GST enzyme immunoassays. Chemico-biological interactions. 1998; 111-112: 123-135.
- Ciba M, Mehmet A, Karahalil B. Alpha-glutathione-s-transferase can be a biomarker for both drug-related toxicity as well as individual susceptibility. 2016.
- Wu H, Xu H, Hong YB, Zhang J, Wu J. The use of biomarkers in the antioxidant responses of Daphnia magna to the acute and chronic exposure to no. 20 diesel oil and 2,4-dichlorophenol. Chemical Speciation & Bioavailability. 2011; 23: 80-87.
- Zitka O, Skalickova S, Gumulec J, Masarik M, Adam V, Hubalek J, et al. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncology letters. 2012; 4: 1247-1253.
- Savic-Radojevic A, Pljesa-Ercegovac M, Matic M, Simic D, Radovanovic S, Simic T. Chapter Four - Novel Biomarkers of Heart Failure. Advances in Clinical Chemistry. GS Makowski. Elsevier. 2017; 93-152.
- Hoffman HN, Phyliky RL, Fleming CR. Zinc-induced copper deficiency. Gastroenterology. 1988; 94: 508-512.
- Broderius M, Mostad E, Wendroth K, Prohaska JR. Levels of plasma ceruloplasmin protein are markedly lower following dietary copper deficiency in rodents. Comparative biochemistry and physiology. Toxicology & pharmacology: CBP. 2010; 151: 473-479.
- Lopez MJ, Royer A, Shah NJJS. Biochemistry, Ceruloplasmin. 2020.
- Johnson MA. COPPER | Physiology In Encyclopedia of Food Sciences and Nutrition (Second Edition), ed. B Caballero. Oxford: Academic Press. 2003. 1640-47.
- Johnson MA. COPPER | Physiology In Encyclopedia of Food Sciences and Nutrition (Second Edition), ed. B Caballero. Oxford: Academic Press. 2003. 1640-47.
- Si M, Lang J. The roles of metallothioneins in carcinogenesis. Journal of Hematology & Oncology. 2018; 11.
- Carvalho CDS, Bernusso VA, Araújo HSSD, Espíndola ELG, Fernandes MN. Biomarker responses as indication of contaminant effects in Oreochromis niloticus. Chemosphere. 2012; 89: 60-69.
- Cousins RJ, Swerdel MR. Ceruloplasmin and Metallothionein Induction by Zinc and 13-cis-Retinoic Acid in Rats with Adjuvant Inflammation 1. Proceedings of the Society for Experimental Biology and Medicine. 1985; 179: 168-172.
- Abbasalipourkabir R, Moradi H, Zarei S, Asadi S, Salehzadeh A, Ghafourikhosroshahi A, et al. Toxicity of zinc oxide nanoparticles on adult male Wistar rats. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2015; 84: 154-160.
- Toffaletti JG. Clarifying the confusion of GFRs, creatinine, and cystatin C. 2018.
- Guo J, Guan Q, Liu X, Wang H, Gleave ME, Nguan CYC, et al. Relationship of clusterin with renal inflammation and fibrosis after the recovery phase of ischemia-reperfusion injury. BMC Nephrology. 2016; 17.
- Khan Z, Pandey M. Role of kidney biomarkers of chronic kidney disease: An update. Saudi journal of biological sciences. 2014; 21: 294-299.
- Waanders F, Timmeren MMV, Stegeman CA, Bakker SJL, Goor HV. Kidney injury molecule-1 in renal disease. The Journal of Pathology. 2010; 220: 7-16.
- Vanmassenhove J, Vanholder R, Nagler E, Biesen WV. Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2013; 28: 254-273.
- McDonald DF, Fagan CJ. Fungus balls in the urinary bladder. Case report. The American journal of roentgenology, radium therapy, and nuclear medicine. 1972; 114: 753-757.
- Liu X, Guan Y, Xu S, Li Q, Sun Y, Han R, et al. Early Predictors of Acute Kidney Injury: A Narrative Review. Kidney and Blood Pressure Research. 2016; 41: 680-700.
- Haggerty GC. Development of Tier I Neurobehavioral Testing Capabilities for Incorporation into Pivotal Rodent Safety Assessment Studies. 1989; 8: 53-69.
- Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 2004; 13: 222-257.
- Moser VC, McDaniel KL, Phillips PM. Rat strain and stock comparisons using a functional observational battery: baseline values and effects of amitraz. Toxicology and applied pharmacology. 1991; 108: 267-283.
- Nikiforov AI, Eapen AK. A 90-Day Oral (Dietary) Toxicity Study of Rebaudioside A in Sprague-Dawley Rats. International Journal of Toxicology. 2008; 27: 65-80.
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. The Journal of biological chemistry. 1951; 193: 265-275.
- Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Analytical biochemistry. 1976; 74: 214-226.
- Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods in enzymology. 1984; 105: 114-120.
- Viarengo A, Ponzano E, Dondero F, Fabbri R. A simple spectrophotometric method for metallothionein evaluation in marine organisms: an application to Mediterranean and Antarctic molluscs. Marine Environmental Research. 1997; 44: 69-84.
- Cheng D, Li H, Zhou J, Wang S. Chlorogenic acid relieves lead-induced cognitive impairments and hepato-renal damage via regulating the dysbiosis of the gut microbiota in mice. Food & function. 2019; 10: 681-690.
- Bailey SA, Zidell RH, Perry RW. Relationships Between Organ Weight and Body/Brain Weight in the Rat: What Is the Best Analytical Endpoint?. Toxicologic Pathology. 2004; 32: 448-466.
- Adkison LR. 10 - Renal, Gastrointestinal, and Hepatic Disorders In Elsevier's Integrated Review Genetics (Second Edition), ed. LR Adkison. Philadelphia: W.B. Saunders. 2012; 177-91.
- Kasperczyk A, Prokopowicz A, Dobrakowski M, Pawlas N, Kasperczyk S. The Effect of Occupational Lead Exposure on Blood Levels of Zinc, Iron, Copper, Selenium and Related Proteins. Biological Trace Element Research. 2012; 150: 49-55.
- Yang H, Yan L, Cao F, Zhao H, Wang Y, Guo X, et al. [Disorder of copper homeostasis induced by lead exposure among mice and intervention effect of quercetin]. Zhonghua lao dong wei sheng zhi ye bing za zhi = Zhonghua laodong weisheng zhiyebing zazhi = Chinese journal of industrial hygiene and occupational diseases. 2013; 31: 759-62.
- Flora SJS, Mittal M, Mehta A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. The Indian journal of medical research. 2008; 128: 501-23.
- Shi R, Huang C, Aronstam RS, Ercal N, Martin A, Huang Y. N-acetylcysteine amide decreases oxidative stress but not cell death induced by doxorubicin in H9c2 cardiomyocytes. BMC Pharmacology. 2009; 9: 7-7.
- Hsieh C, Lu C, Kuo Y, Lin G, Chao C. The protective effect of non-invasive low intensity pulsed electric field and fucoidan in preventing oxidative stressinduced motor neuron death via ROCK/Akt pathway. PLoS ONE. 2019; 14: e0214100.
- Naqvi S, Sharma H, Flora SJS. Lactobionic acid conjugated quercetin loaded organically modified silica nanoparticles mitigates cyclophosphamide induced hepatocytotoxicity. Int J Nanomedicine. 2019; 14: 8943.
- Tapia A, Corripio R, Espinosa M. Variations in the pyocynotype and its epidemiological applications. Revista clinica Espanola. 1971; 122: 303-6.
- Kwon S, Bae O, Noh J, Kim K, Kang S, Shin Y, et al. Erythrophagocytosis of Lead-Exposed Erythrocytes by Renal Tubular Cells: Possible Role in Lead- Induced Nephrotoxicity. Environmental Health Perspectives. 2015; 123: 120- 127.
- Lim AI, Tang SC, Lai KN, Leung JC. Kidney injury molecule-1: More than just an injury marker of tubular epithelial cells?. Journal of Cellular Physiology. 2013; 228: 917-924.
- Gou R, Chen J, Sheng S, Wang R, Fang Y, Yang Z, et al. KIM-1 Mediates High Glucose-Induced Autophagy and Apoptosis in Renal Tubular Epithelial Cells. Cellular Physiology and Biochemistry. 2016; 38: 2479-2488.
- Silva AH, Locatelli C, Filippin-Monteiro FB, Martin P, Liptrott NJ, Zanetti- Ramos BG, et al. Toxicity and inflammatory response in Swiss albino mice after intraperitoneal and oral administration of polyurethane nanoparticles. Toxicology letters. 2016; 246: 17-27.