
Review Article
Austin J Obstet Gynecol. 2024; 11(1): 1226.
Venous Thromboembolism in Pregnancy: A Review Article of Current Best Practice
Raman Dabas; Ayman Aboda*; Brian Mccully
Department of Obstetrics & Gynaecology, Mildura Base Public Hospital, Australia
*Corresponding author: Ayman Aboda Department of Obstetrics & Gynaecology, Mildura Base Public Hospital, Mildura 3500, Victoria, Australia. Email: aymanaboda@hotmail.com
Received: January 03, 2024 Accepted: February 08, 2024 Published: February 15, 2024
Abstract
Venous Thrombo-Embolic Disease (VTE) during pregnancy, including both Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE), is a leading cause of maternal mortality. It contributes to 20% of such deaths in developed countries. The incidence of VTE is estimated at 0.5–2 cases per 1000 pregnancies, with incidents spread across all trimesters, often involving the iliofemoral vessels. The review aims to present the current understanding and recent advancements in managing, diagnosing, and preventing PE and VTE, acknowledging the unique challenges and risks during pregnancy.
Physiological changes in pregnancy, such as hypercoagulability, venous stasis, and vascular injury, increase the risk of VTE, including PE. Diagnosing PE in pregnant women is particularly challenging due to symptom overlap with normal pregnancy changes and risks associated with diagnostic imaging. Treatment most often requires anticoagulant therapy, usually with LMWH and UFH, which are chosen for their efficacy and safety. Management is multidisciplinary and acknowledges individualized care plans, especially post-partum. Ultimately, the goal is to ensure maternal and fetal safety through a nuanced approach to diagnosing and treating PE and VTE during pregnancy.
Introduction
Venous Thrombo-Embolic Disease (VTE), encompassing Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE), is a leading cause of maternal mortality [1]. While it remains a relatively rare occurrence, its impact is profound, contributing to 20% of maternal deaths in developed countries [2-4]. Understanding the prevalence and implications of PE is crucial for clinicians, as it informs both preventive strategies and the management of at-risk pregnancies where the unique physiological changes in pregnancy, such as increased blood coagulability, reduced blood flow from the legs, and potential injury to blood vessels, significantly elevate the risk of thrombotic events [5]. This review aims to provide a comprehensive understanding of the pathophysiology, risk factors, diagnosis, management, and prevention of VTE and PE in the context of pregnancy, highlighting the unique challenges and considerations in this patient population. The increased incidence of VTE during pregnancy is attributed to physiological changes that enhance thrombosis. Elevated levels of prothrombotic factors, such as factors II, VII, VIII, IX, X, fibrinogen, and von Willebrand factor, along with decreased anticoagulant activity and diminished fibrinolysis, create a hypercoagulable state [6]. Progesterone, a pre-eminent pregnancy hormone, relaxes smooth muscle, leading to vasodilation of blood vessels, particularly veins. In the lower extremities, this can lead to stasis or venous pooling, another reason thrombosis may be more likely to occur. In some cases, blood flow is further inhibited by the weight of other organs, most significantly, the gravid uterus or, more rarely, other vessels, as may occur with the May-Thurner syndrome [7,8]. The risk for PE and VTE in pregnancy is heightened by a history of thrombophilia, such as with Factor V Leiden mutation, a genetic disorder inherited from one or both parents that results in an abnormal variant of Factor V, known as Factor V Leiden, which is more resistant to inactivation by protein C, a natural anticoagulant in the blood, leading to an increased tendency to develop blood clots, particularly in the deep veins of the legs and in the lungs [9,10]. Other factors, such as surgery, prolonged immobility, pregnancy, oral contraceptive use, and smoking, can further increase the risk, as can pregnancy-specific conditions such as preeclampsia and caesarean delivery. Factors also include obesity, advanced maternal age, high-order parity, multiple pregnancies, and pre-existing conditions like diabetes and vascular disease [11,12]. Physiological changes in the postnatal factors are particularly critical and can be exacerbated by complications, including operative delivery and bed rest, haemorrhage and sepsis [13]. Diagnosing PE during pregnancy remains challenging due to an overlap of diagnostic symptoms with common pregnancy-related changes, such as shortness of breath and tachycardia [13,14]. Up to 70% of PE patients do not present with DVT at diagnosis, complicating the clinical picture [15,16]. Traditional diagnostic tools, such as the Wells Scale and Geneva score, are not commonly used in pregnancy and other approaches, including the 'LEFT' rule, remain non-specific [17]. Radiological diagnosis is critical; however, utilization must balance the need for diagnosis with the potential exposure risk to the mother and fetus. Venous dopplers, chest X-rays, Ventilation Perfusion (V/Q) scans, and computed tomography pulmonary angiograms CTPA are all employed, with caution, to minimize fetal exposure [18].
In managing PE and VTE during pregnancy, a balance must be struck between the effective treatment of the mother and the safety of the fetus. Anticoagulant therapy, the cornerstone of treatment, must be carefully tailored to mitigate risks while providing effective thromboprophylaxis. This balance requires a nuanced understanding of the available therapeutic options, including the mainstays of treatment: low-molecular-weight heparin (LMWH) and Unfractionated Heparin (UFH). LMWH is preferred due to its efficacy and lower risk profile, while warfarin and newer anticoagulants like dabigatran and rivaroxaban are generally avoided during pregnancy due to their potential risks [19,20].
Current guidelines emphasize the need for individualized treatment plans, effective anticoagulation and a multidisciplinary approach to care for the best possible outcome. The importance of preventive measures and post-partum care, particularly in women with identifiable risk factors, is also highlighted [14,21,22]. In cases of severe symptoms or high-risk PE, treatment options extend to thrombolytic therapy, surgical embolectomy, and catheter-based interventions [14]. The use of Inferior Vena Cava Filters is limited and cautiously approached in pregnant patients. Systemic thrombolysis, though a relative contraindication in pregnancy, is considered in life-threatening situations. The complexity of VTE management is further underscored during labour and delivery, especially when neuraxial anaesthesia is planned. Post-partum, the resumption of anticoagulation is carefully timed to balance thrombotic and haemorrhagic risks [23].
Case Presentation
A 29-year-old primigravidae woman at 38+4 gestation reported spontaneous pre-labour rupture of membranes. On presentation to the birthing suite, she was found to have an incidental tachycardia of 120-150 bpm. She was asymptomatic, with no shortness of breath or chest pain. There was no history of calf pain or URTI, and there was no history of recent travel or surgery. She was on no medications and had no past or family history of VTE disease. Earlier in the first trimester of her pregnancy, her General practitioner had noted tachycardia on a routine antenatal visit. A Holter recording had been normal. Her Antenatal history, including routine investigations, was otherwise normal.
On inspection, the patient was anxious. She could speak in complete sentences. There were no palpitations. A cardiovascular examination found a blood pressure of 116/68 and a regular heart rhythm with a rate varying between 120- 151 beats/min. There were no heart murmurs. There was no peripheral oedema or calf tenderness. Her oxygen saturation was 98% on room air. Respiratory examination was normal, with bilateral air entry and normal breath sounds. Abdominal examination findings were consistent with term pregnancy. A cardiotocogram CTG was normal, and there was no palpable uterine activity. An Amnisure test confirmed rupture of membranes during speculum examination. An ultrasound scan of the pregnancy confirmed appropriate fetal growth with normal cord dopplers and amniotic fluid volume.
An electrocardiogram was performed, which confirmed sinus tachycardia. Chest x-ray was normal. Both lung fields were fully inflated with no evidence of pleural effusion or pneumothorax. Bloods were taken for Full blood count, Urea and electrolytes, Liver function and urate. A CRP and Thyroid function were also requested, as well as a coagulation profile including fibrinogen. These were all normal. A serum Troponin was also normal. She tested negative for COVID, Influenza A, and Respiratory Syncytial Virus RSV.
She had a thoracic computed tomography with intravenous contrast of pulmonary arteries CTPA. She wore lead shielding to minimize radiation exposure to the fetus. The findings demonstrated occlusive filling defects in the anterior right pulmonary artery branches and nonocclusive filling defects in the anterior segmental branches of the left upper lobe pulmonary artery (Figure 1 & 2). There were also several non-occlusive filling defects in the left lower lobe pulmonary artery. Ventilation scans were normal. A diagnosis of bilateral segmental and subsegmental branch pulmonary emboli was made.
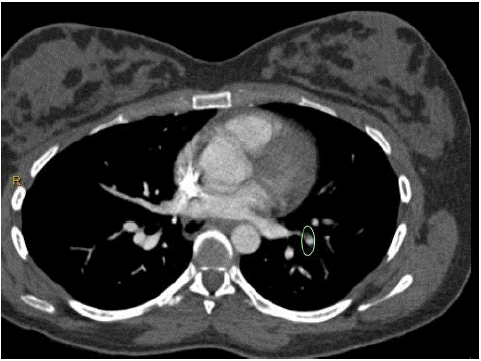
Figure 1:

Figure 2:
The findings were discussed with a tertiary-level Maternal foetal-medicine unit. An on-site multidisciplinary PE Response Team (PERT) was activated, which included haematology, general medicine, anaesthetics, and intensive care physicians. Collaboratively, they recommended expeditious delivery, either by Induction of labour or elective caesarean section, to be followed by therapeutic anticoagulation using unfractionated heparin. The patient requested elective surgical delivery, and this was performed later that evening. The procedure was uncomplicated and resulted in the birth of a healthy baby boy weighing 3140 gms. The estimated blood loss was less than 500 ml. Postoperatively, the patient was transferred to the intensive care unit. Unfractionated heparin infusion was commenced 4 hrs after delivery and continued for 24 hrs. Two hours after starting treatment, the tachycardia resolved completely. After 24 hours, therapeutic Clexane was commenced using subcutaneous enoxaparin sodium, 1 mg/kg every 12 hours.
The patient remained hemodynamically stable with normal oxygen saturation in room air. On day 3, she was transferred to the maternity ward and commenced on warfarin 5 mg with bridging Clexane 60 mg until a therapeutic INR was confirmed in 2 consecutive readings 24 hours apart. She was discharged home on day 5, with follow-up by Hospital in the Home (HITH), until a target INR 2-3 was met. Her warfarin dose was then set at 8 mg daily for three months with follow-up by Haematology.
The patient is now off treatment. It was noted that she had previously used the combined oral contraceptive pill. She has been advised to avoid this indefinitely.
Discussion
Venous Thrombo-Embolic Disease (VTE), which includes Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE), presents a critical challenge in obstetric care due to its increased incidence during pregnancy and its role as a leading cause of maternal mortality [1]. The incidence of VTE in pregnancy is estimated at 0.5–2 cases per 1000 pregnancies, accounting for about 20% of maternal deaths in developed countries [2–4]. This high rate of mortality, particularly in undiagnosed cases, underscores the importance of accurate diagnosis. Up to 70% of PE patients do not exhibit DVT at the time of diagnosis, complicating the clinical picture [14,15]. Understanding the pathophysiology, risk factors, diagnostic challenges, and treatment modalities of VTE in pregnant women is fundamental to managing this condition effectively [5].
Pregnancy induces a hypercoagulable state, increased venous stasis, and a heightened risk of vascular wear and tear leading to injury [24]. Much of this is physiological adaptations to allow increased cardiac output and vascular flow, which are a sine qua non for successful early implantation and placentation and the continued growth support of the ongoing pregnancy [25-27]. The events, however, are intricately linked with susceptibility to abnormal thrombosis, as predicted by Virchow's Triad. Virchow's Triad is a concept in medicine that was developed by the German physician Rudolf Virchow (1821-1902) in the mid-19th century. It represents a foundational understanding of the factors contributing to the formation of blood clots, particularly in veins. Virchow conducted extensive research on the post- mortem examination of patients who had died from various diseases.
During his research, he recognized that thrombosis was not an isolated event but had underlying pathological and physiological predilections. In 1856, he formulated what would later become known as Virchow's Triad, which consisted of three key factors contributing to thrombosis: stasis (changes in blood flow), hypercoagulability (changes in the composition of blood), and endothelial injury (damage to the blood vessel lining). In pregnancy, all components of this Triad are at play. Elevated levels of prothrombotic factors, such as factors II, VII, VIII, IX, X, fibrinogen, and von Willebrand factor, along with decreased anticoagulant activity and diminished fibrinolysis, underscore this hypercoagulable state,8. Progesterone-mediated vasodilation, particularly in the third trimester, contributes to venous stasis by causing blood pooling in the lower limbs and pelvis [27]. Additionally, the gravid uterus can compress the iliac vein, as can, more rarely, the right iliac artery when it overlies and causes narrowing in the left iliac vein. This is called the May-Thurner syndrome, also known as the iliac vein compression syndrome, an anatomical anomaly that can predispose to VTE in the pelvis [28].
Preventive strategies are important, especially for women with known risk factors for VTE. These include obesity, advanced maternal age, high-order parity, multiple pregnancies, and pre-existing conditions like homozygous factor 5 Leiden and vascular disease [29,30]. Supportive management can occur early and may include lifestyle modifications, such as cessation of smoking and alcohol and encouraging moderate exercise and mobility. Preventative or prophylactic thromboprophylaxis may be considered in circumstances of known risk or emergent situations, such as unexpected surgery or hospitalization [14,31]. For all women, the risk of VTE remains high in the post-partum period, particularly if birth is complicated by instrumental or operative deliveries such as caesarean section, haemorrhage or sepsis [32]. This risk may persist for several weeks post-partum, necessitating ongoing vigilance [13,20].
Diagnosing PE in pregnant women presents unique challenges due to the overlapping symptoms with common pregnancy-related changes, such as shortness of breath, chest pain, and palpitations, leading to potential underdiagnosis or misdiagnosis [6,14,15]. Standard diagnostic scores like the Wells Scale and Geneva clinical scoring systems help determine the likelihood of a patient having a PE and guide decisions regarding further diagnostic testing and treatment [16]. The criteria include clinical signs and symptoms, such as leg swelling, dyspnoea, chest pain, abnormal HR and BP, and risk factors, such as previous DVT or protracted immobilization [16]. Both, however, lack validation in pregnant women. The "LEFT" clinical decision rule may be used to evaluate patients suspected of having a lower extremity Deep Vein Thrombosis (DVT). It includes leg swelling, Oedema (spelt edema), the first episode, which suggests that the findings represent a sudden event, and finally, T for Tenderness. If two or more criteria are present, the patient is at high risk, and further investigation should follow [33-35].
The use of diagnostic imaging, such as X-ray or CT pulmonary angiography, is confounded by concerns about fetal radiation exposure. While this remains a counterpoint mediating all decisions regarding investigative imagining, we must remember that a well and effectively managed mother is the best protection for a foetus. Venous dopplers, chest X-rays, V/Q scans, and Computed Tomography Pulmonary Angiograms (CTPA) are commonly used, with precautions taken to minimize fetal exposure [12-15]. Once diagnosed, the primary goal is to treat the mother effectively without jeopardizing foetal well- being and safety. Anticoagulant therapy is the mainstay of pharmacological treatment. The choice of agent must be carefully tailored to the drug's safety profile, efficacy, and the stage of pregnancy. Low Molecular Weight Heparin (LMWH) is commonly preferred due to its efficacy and safety profile in pregnancy [12,13,18,19]. It does not cross the placenta and is generally chosen over UFH because of its lower risk of heparin-induced thrombocytopenia and reduced risk of bleeding. The duration and intensity of treatment are individualized depending on the patient's risk factors and the likely sequelae of the identified disease [12,13,18,19]. Additional therapies include graduated elastic compression stockings and early mobilization [36,37]. Research into newer anticoagulants is ongoing. For high-risk patients or those with life-threatening symptoms, more aggressive interventions like thrombolytic therapy, surgical embolectomy, and catheter-based treatments are considered [38,39]. Inferior Vena Cava Filters in pregnancy have been rarely reported [40,41]. Systemic thrombolysis, though effective, is used cautiously due to the relative contraindication in pregnancy and the risk of significant bleeding [23].
The take-home message for critical active care is to individualize decisions based on a collaborative, multidisciplinary approach involving obstetricians, haematologists, other specialists, and the patient to empower rapid, effective, evidence-based care with continuous surveillance of effect.
Conclusion
Venous Thrombo-Embolic Disease (VTE), including Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE), in pregnancy, poses a significant challenge in maternal healthcare. The physiological changes of pregnancy, such as increased coagulability, venous stasis, and potential endothelial injury, substantially raise the risk of thrombotic events like PE. Understanding these elements is crucial for timely diagnosis, effective treatment, and prevention of PE in pregnant women. The management of VTE in pregnancy requires a collaborative, multidisciplinary approach, considering each patient's unique risk profile, the stage of pregnancy, and the balance between thrombotic and bleeding risks. The management of VTE, particularly PE, during pregnancy centres around balancing effective maternal treatment with fetal safety. Anticoagulant therapy, predominantly with LMWH and UFH, is the cornerstone of treatment, demanding careful consideration of the drug's safety profile, efficacy, and stage of pregnancy. Preventive strategies complement this, crucial for women with identifiable risk factors, and the sustained vigilance required in post-partum care due to the continued risk of VTE, leading to safer and more effective obstetric care.
References
- Teresa Pagano, Irma Sofia Fabbri, Marcello Benedetto, Luca D’Angelo, Giorgio Galizia, et al. Predicting in-hospital mortality in patients admitted from the emergency department for pulmonary embolism: Incidence and prognostic value of deep vein thrombosis. A retrospective study. 2023.
- Frida Lonnberg, Andreas Roos, Maria Farm, André Heurlin, Mantas Okas, et al. Causes of death after first-time venous thromboembolism. 2023.
- Hunter Mwansa, Mohamed Zghouzi, Geoffrey Barnes. Unprovoked Venous Thromboembolism: The Search for the Cause. 2023; 107: 861-882.
- Marco Zuin, Behnood Bikdeli, Andre Armero, Gianluca Rigatelli, Claudio Biloto, et al. Trends in Pulmonary Embolism Deaths Among Young Adults Aged 25 to 44 Years in the United States, 1999 to 2019. The American Journal of Cardiology. 2023; 202: 169-175.
- Hun-Gyu Hwang, Ju Hyun Lee, Soo-Mee Bang. Incidence of Pregnancy-Associated Venous Thromboembolism: Second Nationwide Study. Thromb Haemost. 2023; 123: 904-910.
- Dimitrios Varrias, Michail Spanos, Damianos G Kokkinidis, Panagiotis Zoumpourlis, Dimitrios Rafail Kalaitzopoulos. Venous Thromboembolism in Pregnancy: Challenges and Solutions. Vascular Health and Risk Management. 2023; 19: 469-484.
- Update on pregnancy-associated venous thromboembolism Arielle L. Langer, Nathan T. Connell Thrombosis Update Volume 8, August 2022, 100107.
- Felis S, Marchese B, Gavini I. Venous Thromboembolism During Pregnancy Med Clin Res. 2023; 8: 01.
- Bitsadze V, Khizroeva J, Alexander M, Elalamy I. Venous thrombosis risk factors in pregnant women. From the journal Journal of Perinatal Medicine. 2022.
- Laila F Zahed, Roni F Rayes, Rami A Mahfouz, Ali T Taher, Huda H Maarouf, et al. Prevalence of factor V Leiden, prothrombin and methylene tetrahydrofolate reductase mutations in women with adverse pregnancy outcomes in Lebanon. American Journal of Obstetrics and Gynecology. 2006; 195: 1114-1118.
- Daniele Pastori, Vito Maria Cormaci, Silvia Marucci, Giovanni Franchino, Francesco Del Sole, et al. A Comprehensive Review of Risk Factors for Venous Thromboembolism: From Epidemiology to Pathophysiology. Int J Mol Sci. 2023; 24: 3169.
- Gudisa Bereda. Risk Factors, Diagnosis, Pathophysiology and Management of Deep Vein Thrombosis. J Clin Med Img Case Rep. 2022; 2: 1200.
- Brandon C Maughan, Maria Marin, Justin Han, Karen J Gibbins, Anupama G Brixey, et al. Venous Thromboembolism during Pregnancy and the Post-partum Period: Risk Factors, Diagnostic Testing, and Treatment. Obstet Gynecol Surv. 2022; 77: 433–444.
- Barry Kevane, Fionnuala Ní Áinle. Prevention, diagnosis, and management of PE and DVT in pregnant women. Hematology Am Soc Hematol Educ Program. 2023; 2023: 237–247.
- S Borekci, IK Oguzulgen, SV Konstantinides. Pulmonary Embolism: Diagnosis and Treatment Strategies. Airway diseases. Springer. 2023.
- C Falster, M Hellfritzsch, TA Gaist, M Brabrand, R Bhatnagar, M Nybo, et al. Comparison of international guideline recommendations for the diagnosis of pulmonary embolism. The Lancet. 2023.
- Efficacy evaluation of clinical prediction rules to reduce diagnostic time of venous thromboembolism in the emergency department: a quasi-experimental study A Taberner Balaguer, Anna. 2020.
- DR Kalaitzopoulos, A Panagopoulos, S Samant, N Ghalib, J Kadillari, A Daniilidis, et al. Management of venous thromboembolism in pregnancy. Thrombosis research. 2022; 211: 106-113.
- Abbattista Mariaa, Capecchi Marco, Gianniello Francescaa, Artoni Andreaa, Bucciarelli Paoloa, et al. A retrospective study on the use of low-molecular-weight heparin for prevention of pregnancy-related recurrent venous thromboembolism. Blood Coagulation & Fibrinolysis. 2023; 34: 111-117.
- Ingrid M, Buchmüller Andrea, Wiegers Hanke MG, Áinle Fionnuala Ní, Tardy Bernard, et al. Intermediate-Dose Versus Low-Dose Low-Molecular-Weight Heparin in Pregnant and Post-Partum Women with a History of Venous Thromboembolism (Highlow Study): An Open-Label, Multicentre, Randomised, Controlled Trial Bistervels, Obstetrical & Gynecological Survey. 2023; 78: 259-261.
- Somaya Ouda Abd ELmoniem, Aziza Fathy EL Sayed, Afaf Mohamed Mohamed Emam. Effect of Implementing Evidence-Based Nursing Practices Guidelines on Prevention of Deep Venous Thrombosis among Post-partum Women. Egyptian Journal of Health Care. 2023; 14: 611-631.
- Shimaa Mohamed Hashem, Ekbal Ebrahim Abdelmenem, Faiza Mohamed EL-Said. Effect of Nursing Intervention on Nurses’ Performance Regarding the Prevention of Deep Vein Thrombosis among Post-partum Women. Tanta Scientific Nursing. 2023; 31: 318-341.
- VT Ho, A Dua, K Lavingia, Rothenberg K, Rao C, et al. Thrombolysis for venous thromboembolism during pregnancy: A literature review. Vascular and Endovascular Surgery. 2018; 52: 527-534.
- D Varrias, M Spanos, DG Kokinidis, P Zoumpouris, DR Kalaitzopoulos. Venous Thromboembolism in Pregnancy: Challenges and Solutions Vascular Health and Risk Management. 2023; 19: 469-484.
- M Diop, K Bianco, A Khandelwal. Normal Physiology of Pregnancy and labour Contemporary Topics in Cardio-Obstetrics. Springer. 2023; 25-38.
- M Perales, TS Nagpal, R Barakat. Physiological Changes During Pregnancy: Main Adaptations, Discomforts, and Implications for Physical Activity and Exercise. Exercise and Physical Activity During Pregnancy and Post-partum. 2022; 47-59.
- S Somani, S Rathi, R Ekka. A Comparative Study of Cardiovascular Parameters in Different Trimester of Pregnancy. European Journal of Cardiovascular Medicine. 2023; 13: 663-668.
- G Speranza, M Sadek, G Jacobowitz. Common iliac vein stenting for May-Thurner syndrome and subsequent pregnancy. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2022; 10: 348-352.
- S Middeldrop, R Nieuwlaat, L B Kreuziger, M Coppens, D Houghton, et al. American Society of Hematology 2023 guidelines for the management of venous thromboembolism: thrombophilia testing. Blood Advances. 2023; 7: 7101-7138.
- M Nicholson, N Chan, V Bhagirath and J Ginsberg. Prevention of venous thromboembolism in 2020 and beyond Journal Clinical Medicine. 2020; 9: 2467
- E Schapkaitz, E Libhaber, Gerber A, Rhemtula H, Zamparini J, et al. longitudinal Study of Thrombosis and Bleeding Outcomes with Thromboprophylaxis in Pregnant women at Intermediate and High Risk of VTE. Clinical and Applied Thrombosis/Hemostasis. 2023.
- M Blondon, L Skeith. Preventing post-partum venous thromboembolism in 2022: A narrative review. Frontiers in Cardiovascular Medicine. 2022; 9.
- R Chopard, IE Albertsen, G Piazza. Diagnosis and treatment of lower extremity venous thromboembolism: a review. JAMA. 2020; 324: 1765-1776.
- M Bhatt, C Braun, P Patel, P Patel, H Begum, Wiercioch W, et al. Diagnosis of deep vein thrombosis of the lower extremity: a systematic review and meta-analysis of test accuracy. Blood Adv. 2020; 4: 1250–1264.
- ND Romo. Deep Venous Thrombosis.
- Benefits of early mobilization in patients with deep venous thrombosis: a scope review AR de Alencar Martins, LER Junior.
- E Rabe, F Pannier, JD Milic. The role of compression in deep vein thrombosis, post-thrombotic syndrome prevention and treatment. 2023.
- M Ismayl, A Ismayl, D Hamadi, A Aboeata. Catheter-directed thrombolysis versus thrombectomy for submassive and massive pulmonary embolism: A systematic review and meta-analysis. Cardiovascular Revascularization Medicine. 2023.
- A Azari, AT Beheshi, Z Moravvej, L Bigdelu, M Salehi. Surgical embolectomy versus thrombolytic therapy in the management of acute massive pulmonary embolism: Short and Long-term prognosis. Heart & Lung. 2015; 44: 33-339.
- IM Bistervels, AE Geerlings, PI Bonta. Pregnancy in Women with an Inferior Vena Cava Filter: A Tertiary Centre Experience and overview of the literature. Blood Adv. 2021; 5: 4044-4053.
- F Zhang, J GU, HL Li, X Li, DH Ji, JH Huang. Diagnosis and treatment of venous thromboembolism and clinical application of inferior vena cava filter in China. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2023; 11: 1149-1156.