
Research Article
Int J Nutr Sci. 2023; 8(2): 1074.
Protective Effects of Melatonin on Hepatic Injury Due to Chronic Monosodium Glutamate Consumption in Adult Female Rats
Selin Akbulut1,2; Hilal Nisva Levent1,3; Sevil Arabaci Tamer4,5; Berrak Caglayan Yegen4; Serap Sirvanci1*
1Marmara University, School of Medicine, Department of Histology and Embryology, Istanbul, Turkey
2Sanliurfa Education and Research Hospital, Spermiogram Unit, Sanliurfa, Turkey
3Kartal Lutfi Kirdar Education and Research Hospital, In Vitro Fertilization Unit, Istanbul, Turkey
4Marmara University, School of Medicine, Department of Physiology, Istanbul, Turkey
5Sakarya University, School of Medicine, Department of Physiology, Sakarya, Turkey
*Corresponding author: Serap Sirvanci Marmara University, School of Medicine, Department of Histology and Embryology, Istanbul, Turkey. Email: ssirvanci@marmara.edu.tr
Received: May 31, 2023 Accepted: June 28, 2023 Published: July 05, 2023
Abstract
In the present study, it was aimed to investigate the effects of Monosodium Glutamate (MSG) consumption on rat liver and the possible antioxidant and anti-inflammatory effects of melatoninin protecting against MSG-induced hepatic damage. Thirty two adult female rats were randomly divided into 4 groups. For 30 days, MSG (2g/kg/day) or melatonin (4mg/kg/day) was given daily in drinking water or both melatonin and MSG were given simultaneously at the same doses, while normal drinking water was given to the control group. Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) levels were investigated in the serum of rats decapitated at the end of the experiment. In liver sections, Hematoxylin-Eosin (H-E), Sirius red, Periodic Acid Schiff (PAS) staining for histopathological damage examination, Nuclear Factor-kappa B (NF-κB), smooth muscle a-actin [alpha-Smooth Muscle Actin (a-SMA)], NADPH Oxidase (NOX-2), Transforming Growth Factor (TGF)-β1, SMAD2, SMAD7 immunohistochemistry were applied. At the end of the experiment, % body weight change in MSG group rats was high, and serum ALT and AST levels were increased compared to control group, while these levels weredecreased with melatonin administration. In addition, vacuolization in hepatocytes, dilatation in sinusoids, increase in Kupffer cells andinflammatory cell foci were detected in the MSG group. When melatonin wasadded to MSG, decreased hepatocyte glycogen content and SMAD7 immunoreactivity with increased a-SMA were observed along with improvements in NF-kB, NOX-2, TGF-β1, SMAD2 immunoreactivity. Our findings suggest that melatonin, with its antioxidant and anti-inflammatory effects, may be a potential agent to prevent liver damage that can be caused by MSG in the diet.
Keywords: Monosodium glutamate; Liver; Melatonin; Oxidative stress; Inflammation
Introduction
Today, with the advancement in food technology and change in dietary habits, production and consumption of processed, packaged, and ready-to-eat food are increased. Monosodium Glutamate (MSG) is one of the food additives, which is widely used as a flavor enhancer [1,2]. Although these substances have several benefits and functions in terms of food industry, their effects on human health are controversial. Following its absorption, MSG is metabolized in the liver and the liver is one of the most vulnerable organs to toxic insults [3]. In various animal models using MSG, it was reported that MSG is toxic to the liver, brain, thymus, ovary, tuba uterina, testes, kidneys, and hematopoietic system [4]. Toxic effects of MSG application was associated with increased lipid peroxidation, decreased glutathione levels, and decreased catalase and superoxide dismutase activities in the tissues [5]. Oral application of MSG at the doses which is thought suitable for humans, caused increase in liver oxidative stress markers [6]. Besides, it was also reported that its application resulted in obesity, diabetes, steatosis in hepatocytes, inflammation, fibrosis, neoplastic changes, nodular lesions and degeneration in biliary ducts [6,7]. In a study in which rat pups were fed with MSG for 1 year starting from neonatal period; obesity, increase in serum lipid profiles, deteriorated glucose tolerance and metabolic syndrome was observed [8]. Even MSG was given orally, increased insulin levels were observed within 3 minutes [9]. Another study using MSG application reported hyperinsulinemia, obesity, adipocyte dysfunction and related reproductive system disorder [10]. MSG was reported to cause toxic effects on the liver and costic effects on gastrointestinal tract epithelium [11].
Melatonin is a hormone which has a role in sleep cycle, regulation of circadian rhyhm, and regulation of many biological functions such as reproduction and immunity [12]. It has been reported in different studies that antioxidant and antiinflammatory effects of melatonin might be useful for ameliorating hepatotoxicity caused by toxic agents [13,14].
In the present study, it was aimed to investigate possible protective effects of melatonin on the liver injury caused by MSG ingestion, by morphological and biochemical methods.
Materials And Method
Animals
Three-month-old adult female Wistar albino rats were used in the present study. Animals were obtained from Marmara University, The Experimental Animal Implementation and Research Center. All experiments were done according to the National Guidelines on Animal Experimentation and were approved by the Marmara University Local Ethical Committee for Experimental Animals (43.2021.mar). The animals were housed in a 12-hr light/dark cycle and humidity controlled room.
Groups were as follows:
Control group (n=8): Animals were given normal drinking water for 1 month.
Melatoningroup (n=8):4 mg/kg/daymelatonin was added to drinking water for 1 month.
MSG group(n=8):2 g/kg/day MSG was added to drinking water for 1 month [15].
MSG+melatonin group (n=8):2 g/kg/day MSG and 4 mg/kg/daymelatonin were added to drinking water for 1 month.
Chemicals
In the present study, MSG with =98.0% purity was used (L-glutamic acid monosodium salt monohydrate, Sigma-Aldrich, USA, 49621). Average water consumption of each rat in one day was calculated as approximately 10-12ml/100g. MSG was daily prepared and given in drinking water as 2g/100ml according to the animal’s body weight. Thus, each rat was given MSG 2g/kg/day.
Melatonin was used as =98.0% purity level (Sigma-Aldrich, USA, M5250). It was given in drinking water as 4mg/100ml.
Experiment Protocol
Weight of the animals was measured at the beginning and the end of the study. The percentage of weight loss or weight gain of each animal was calculated [(weight change/first weight)x100]. Lee index was used for evaluation of obesity [16]. Values >310 were accepted as obesity.
At the end of the experiments, rats were deeply anesthetized with ketamine (100mg/kg) and xylazine hydrochloride (10mg/kg), intracardiac blood samples were obtained and then they were decapitated.
Biochemical Evaluation
Blood samples obtaned from decapitated animals were centrifuged at 3000rpm for 10 min. Serum and liver tisues were kept at -20°C until biochemical evaluations were done. Alanine Aminotransferase (ALT) and aspartate transaminase levels in serum were detected by using commercial kits with an autoanalyzer (Human/300) in Vetlab Veterinary Diagnostics Laboratory.
Light Microscopic Preparation and Histological Scoring
Liver tissues were fixed in 10% neutral buffered formalin. After dehydration in ascending series of ethanol, tissues were cleared in xylene. Tissues were embedded in paraffin after incubating in liquid paraffin at 60°C overnight. Four-micron-thick sections were stained with Hematoxylin and Eosin (H&E) for histopathological evaluation and with Sirius red for collagen density. PAS reaction was applied for evaluating glycogen content in hepatocytes. Sections were examined under an Olympus DP72 camera attached Olympus BX51 photomicroscope (Tokyo, Japan).
Five randomly selected areas in H&E stained sections under X20 objective were examined. Vacuolization in hepatocytes, vasocongestion, sinusoidal dilatation and Kupffer cell infiltration were evaluated. Histopathological findings were scored as follows: 0, no pathological finding; 1, mild; 2, moderate; 3, severe pathological findings [17]. Five areas in the sections stained with Sirius red were examined under X40 objective and evaluated by using Image J program; and percentage of stained areas were calculated. Glycogen content in sections stained with PAS was scored as 0, no staining; 1, mild; 2, moderate; 3, heavy staining [18].
Immunohistochemistry
Liver sections were immunostained for a-SMA, NF-κB, NOX-2, TGF-β1, Smad2, and Smad7. Four-micron-thick sections were incubated in 37°C and then deparaffinized in xylene. Sections were incubated in pure ethanol, 96% ethanol, and in 3% H2O2 in methanol for blockage of endogenous peroxidase activity. For antigen retrieval, sections were exposed to citrate tampon in microwave and incubated in blocking solution. Sections were incubated in primary antibodies (a-SMA, Abcam, ab7817, 1:200; NF-κB, Santa Cruz, sc-109, 1:100; NOX-2, Bioss, bs-3889R, 1:200, TGF-β1, Santa Cruz, sc-52893, 1:100; Smad2, Santa Cruz, sc-101153, 1:200; Smad7, Santa Cruz, sc-101152, 1:200) at 4°C. Then, biotinylated secondary antibody (SHP125, ScyTek Laboratories, Inc. USA) and Horse-Radish Peroxidase (HRP) solution were applied. After applying DAB chromogen (DAB Chromogen/Substrate Bulk Pack, ACK500, ScyTek Laboratories, Inc. USA), sections were counterstained with Mayer’s hematoxylin and mounted with Entellan. For negative controls, PBS was used instead of primary antibodies. Randomly selected five areas were photographed under X40 objective and percentage of NF-κB,a-SMA, NOX-2, TGF-β1, Smad2 and Smad7 positive areas were evaluated by using Image-J program.
Statistical Analysis
Data were analyzed by using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA) program and presented as mean±S.E.M. One-Way-ANOVA and Tukey multiple comparison tests were used. p<0.05 was considered as significant.
Results
Weight Follow-upand Calculation of Lee Index
Weight gain in the animals in MSG group was increased compared to the control group (p<0.001). Weight gain in MSG+melatonin (p<0.01) and melatonin (p<0.0001) groups were significantly decreased compared to MSG group (Figure 1a). Lee index score was increased in MSG group compared to the control group (p<0.0001), but decreased in melatonin (p <0.0001) and MSG+melatonin (p<0.01) groups as compared to the MSG group (Figure 1b).

Figure 1: (a) Percentage of weight change. ***: p<0.001, compared to the control group; ++++: p<0.0001, ++: p<0.01 compared to the MSG group. (b) Lee index score. ****: p<0.0001, compared to the control group; ++: p<0.01, ++++: p<0.0001,compared to the MSG group. (c) Serum ALT levels. **: p<0.01, compared to the control group; +: p<0.05, compared to the MSG group. (d) Serum AST levels. **: p<0.01, compared to the control group; ++: p<0.01, +: p<0.05, compared to the MSG group.
Biochemical Evaluation
ALT and AST levels were increased in MSG group compared to the control group (p<0.01); while both levels were decreased in MSG+melatonin group compared to the MSG group (p<0.05) (Figure 1c, d).
Histopathological Findings
Hepatocytes and sinusoids showed normal morphology, and Kupffer cell number was normal in control (Figure 2a) and melatonin (Figure 2b) groups. Vacuolization in hepatocytes, sinusoidal dilatation, vasocongestion in portal area and increased number of Kupffer cells were observed in MSG group (Figure 2c). The number of vacuolized hepatocytes and Kupffer cells, vasocongestion and sinusoidal dilatation were decreased in MSG+melatonin group (Figure 2d). Histopathological score was increased in MSG group compared to the control group (p<0.001), and decreased in MSG+melatonin group compared to the MSG group (p<0.001) (Figure 2e).
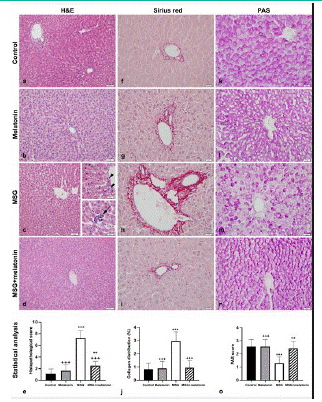
Figure 2: Normal liver morphology in control (a) and melatonin (b) groups. (c) Sinusoidal dilatation is evident in MSG group. Upper inset: Vacuoles (arrowhead) in hepatocytes. Lower inset: Inflammatory cell infiltration (arrow). (d) Normal liver morphology in MSG+ melatonin group. (e) Histopathological score. ***: p<0.001, **: p<0.01, compared to the control group; +++: p<0.001, compared to the MSG group. Collagen distribution in control (f), melatonin (g), MSG (h), and MSG+melatonin (i) groups. Increased collagen tissue is evident in MSG group. (j) Percentage of average collagen distribution. ***: p<0.001, compared to the control group; +++: p<0.001, compared to the MSG group. Glycogen distibution in control (k), melatonin (l), MSG (m), and MSG+melatonin (n) groups. Decreased glycogen in hepatocytes is evident in MSG group. (o) Semi-quantitative assessment of glycogen distribution. ***: p<0.001, compared to the control group; +++: p<0.001, ++: p<0.01, compared to the MSG group. (a-d) Hematoxylin and eosin staining, (f-i) Sirius red, (k-n) PAS reaction. Bars: 50 μm. Bars in insets: 20 μm.
The presence of collagen fibers in liver parenchyma and periportal areas was scant in control (Figure 2f) and melatonin (Figure 2g) groups. Increased collagen density was observed especially in periportal areas, and collagen distribution was slightly widespread in MSG group (Figure 2h). Collagen accumulation in parenchyma and periportal areas was decreased in MSG+melatonin group (Figure 2i). The percentage of collagen distribution was increased in MSG group compared to the control group (p<0.001) and decreased in MSG+melatonin group compared to the MSG group (p<0.001) (Figure 2j).
In PAS-stained sections, glycogen distribution was normal in control (Figure 2k) and melatonin (Figure 2l) groups; however, it was impaired in MSG+melatonin group (Figure 2m). Glycogen distribution was similar to the control group in MSG+melatonin group (Figure 2n). PAS staining score was decreased in MSG group compared to the control group (p<0.001) and increased in MSG+melatonin group compared to the MSG group (p<0.01) (Figure 2o).
Immunohistochemistry
a-SMA Immunohistochemistry
The percentage of a-SMA stained regions in portal areas and around the central vein was less in control (Figure 3a) and melatonin (Figure 3b) groups. MSG group showed more a-SMA stained areas compared to the control group (p<0.0001) (Figure 3c). MSG+melatoningroup showed less stained areas compared to the MSG group (p<0.0001) (Figure 3d, 3e).
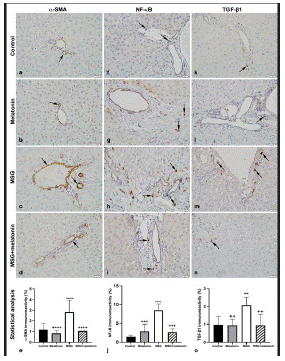
Figure 3: a-SMA immunoreactivity (arrows) in control (a), melatonin (b), MSG (c), and MSG+melatonin (d) groups. (e) Percentage of a-SMA immunoreactive areas. ****: p<0.0001, compared to the control group; ++++: p<0.0001, compared to the MSG group. NFκB immunoreactive cells (arrows) in control (f), melatonin (g), MSG (h), and MSG+melatonin (i) groups. (j) Percentage of NFκBimmunoreactivity. ***: p<0.001 compared to the control group; +++: p<0.001, compared to the MSG group. TGF-β1 immunoreactive cells (arrows) in control (k), melatonin (l), MSG (m), and MSG+melatonin (n) groups. (o) Percentage of TGF-β1 immunoreactivity.**: p<0.01, compared to the control group; ++: p<0.01, compared to the MSG group.
NF-κB Immunohistochemistry
A few NF-κB positive cells were observed in control (Figure 3f) and melatonin (Figure 3g) groups. NF-κB positive cells were numerous in MSG group (Figure 3h) and less in MSG+melatonin group (Figure 3i). The percentage of NF-κBimmunoreactive cell areas was increased in MSG group compared to the control group (p<0.001) and decreased in MSG+melatonin group compared to the MSG group (p<0.001) (Figure 3j).
TGF-β1 Immunohistochemistry
TGF-β1 immunoreactivity was less in the portal areas of control (Figure 3k) and melatonin (Figure 3l) groups. TGF-β1 immunorective cells in in portal areas and parenchyma of MSG group (Figure 3m) was increased compared to the control group. TGF-β1 immunostaining in MSG+melatonin group was similar to the control group (Figure 3n). The percentage of TGF-β1 immunoreactive cell areas was increased in MSG group compared to the control group (p<0.01) and decreased in MSG+melatonin group compared to the MSG group (p<0.01) (Figure 3o).
Smad2 Immunohistochemistry
Only a few Smad2 immunopositive cells were observed in control (Figure 4a) and melatonin (Figure 4b) groups. Immunopositive cells in parenchyma and portal areas were increased in MSG group (Figure 4c). Immunopositivity was similar in MSG+melatonin group (Figure 4d) was similar to that of control group. The percentage of Smad2 positive areas in MSG group was significantly increased compared to the control group (p<0.0001) and decreased in MSG+melatonin group compared to the MSG group (p<0.0001) (Figure 4e).
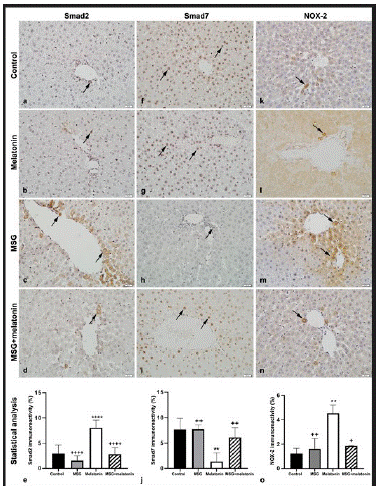
Figure 4: Smad2 immunoreactive cells (arrows) in control (a), melatonin (b), MSG (c), and MSG+melatonin (d) groups.(e) Percentage of Smad2 immunoreactivity. ****: p<0.0001, compared to the control group; ++++: p<0.0001, compared to the MSG group. Smad7 immunoreactive cells (arrows) in control (f), melatonin (g), MSG (h), and MSG+melatonin (i) groups. (j) Percentage of Smad7 immunoreactivity. **: p<0.01, compared to the control group; ++: p<0.01, compared to the MSG group. NOX-2 immunoreactive cells (arrows) in control (k), melatonin (l), MSG (m), and MSG+melatonin (n) groups. Percentage ofNOX-2 immunoreactivity. **: p<0.01, compared to the control group; ++: p<0.01, +: p<0.05, compared to the MSG group.
Smad7 Immunohistochemistry
Numerous Smad7 immunoreactive cells were observed in control (Figure 4f) and melatonin (Figure 4g) groups. Immunopositive cells were decreased in MSG group (Figure 4h) and immunoreactivity in MSG+melatonin group (Figure 4i) was similar to that of the control group. The percentage of Smad7 immunoreactve areas were significantly decreased in MSG group compared to the control group (p<0.01) and increased in MSG+melatonin group compared to the MSG group (p<0.01) (Figure 4j).
NOX-2 Immunohistochemistry
A few NOX-2 immunopositive cells were observed in control (Figure 4k) and melatonin (Figure 4l) groups. Immunoreactive cells were increased in MSG group compared to the control group (Figure 4m). MSG+melatonin group (Figure 4n) showed similar immunoreactivity with that of the control group. The percentage of NOX-2 immunoreactive areas was increased in MSG group compared to the control group (p<0.01) and decreased in MSG+melatonin group compared to the MSG group (p<0.01) (Figure 4o).
Discussion
In the present study, MSG application in rats resulted in obesity and increases in serum ALT and AST levels. Besides, vacuolization in hepatocytes, vasocongestion, increased Kupffer cells and inflammatory cells, and increased collagen fibers in MSG group were observed. These findings were supported by a-SMA, NF-κB, NOX-2, TGF-β1, Smad2 and Smad7 immunohistochemistry results. All these parameters in the liver tissues of MSG+melatonin group were similar to those of the control group.
Lee index greater than 310 is a determinant of obesity and in our study we observed that animals in the MSG group had obesity according to the Lee index. The value of Lee index in MSG+melatonin group was slightly higher than 310 and in control and melatonin groups it was lower than 310. These changes in MSG group in the present study are in line with the previous studies. In a previous study, subjects were asked to prepare their daily meals at home by themselves and the amount of the meal be the same [19]. When the subjects of whom the meals were added MSG and not added were compared, it was observed that MSG caused obesity independent of physical activity and energy amount taken. Another study investigated the effects of MSG on the fetal brain development taken in late gestational period in mice. In the animals given MSG, weight gain ratio in the postnatal 90th day was much higher compared to the control group [20]. Increased body fat deposition after pinealectomy in rats was observed to decrease after melatonin treatment; also, insulin and glucose values were returned to normal levels [21]. In the present study, the findings caused by MSG intake were reversed and returned to control levels after melatonin treatment. Obesity is associated with nonalcoholic fatty liver disease, characterized by intrahepatic steatosis with or without steatohepatitis and fibrosis [22]. Melatonin prevents liver steatosis in obesity and it is important to prevent the risk of nonalcoholic fatty liver disease [23-24].
It was shown that MSG application in rats for 14 days caused increases in AST and ALT levels [25]. It was reported that MSG caused hepatotoxic effect even in small doses; therefore, it was suggested not to use MSG in liver diseases. Studies have demonstrated that MSG increased serum ALT activity by inducing oxidative stress [5,26]. On the other hand, serum AST, ALT, total and conjugated bilirubin levels were increased after liver injury caused by CCl4 and these values significantly decreased after melatonin treatment [27]. Besides, increased AST and ALT levels caused by inflammation after high fat diet were significantly decreased after melatonin treatment [28]. Similarly, in our study, serum AST and ALT levels were significantly increased in MSG group, and the levels have returned to control values in MSG+melatonin group.
In a previous study it was shown that high dose MSG caused deterioration in liver morphology, central vein dilatation, degeneration and atrophy in hepatocytes [29]. Another study reported central vein dilatation, various disorders of hepatocyte structure, apoptosis and fibrosis in the rats fed with MSG [30]. MSG given to rats caused cytoplasmic vacuolization, swelling of mitochondria, vesicular endoplasmic reticulum, pyknotic nuclei, and decrease in carbohydrate and protein deposits [7]. Other studies showed similar injury findings in the liver tissue [29,31-34]. Similarly, in our study, normal liver morphology observed in control groups was deteriorated in MSG group. Hepatocellular vacuolization, sinusoidal dilatation, Kupffer cell number and inflammatory foci were decreased in melatonin-treated group compared to the MSG group. Many studies using toxic agents reported the ameliorating effect of melatonin on liver histopathology [35,36]. This protective effect against injury was related to the cytoprotective and antioxidant effects and inhibition of neutrophil infiltration, necrosis and apoptosis [36].
In the present study, glycogen deposition in hepatocytes was significantly decreased in MSG group, and it returned to control levels with melatonin treatment. This decrease was reported to be due to enzyme modifications in glycolytic pathway [32]. Glucose metabolism with MSG application was reported to change into lipogenesis, resulting in decrease in glycogen deposition [37].
Liver fibrosis develops as a response to hepatocyte injury and it is characterized by excessive matrix synthesis and disorder in matrix destruction [38]. Stellate cells were reported to be the main cells responsible for excess collagen production [38,39]. In a previous study in newborn mice with nonalcoholic steatohepatitis caused by MSG injection, fibrotic hepatic parenchyma and dysplastic nodules were observed [6]. Melatonin was reported to demonstrate its effect on fibrosis by inhibiting stellate cells and inflammatory cell infiltration, by preventing oxidative stress [40]. It was also reported that melatonin could inhibit oxidative stress and provide protection against hepatic fibrosis induced by dimethylnitrosamine [41]. In another study in which liver fibrosis was induced by CCl4, melatonin was demonstrated to protect against fibrosis by mitophagy and upregulation of mitochondrial biogenesis; and it was suggested that it could be a useful agent in antifibrotic treatment [42]. It was shown in a previous study that melatonin prevented liver fibrosis by inhibiting TGF-β1/Smad pathway [43]. In line with the previous studies, in the present study, collagen deposition in liver parenchyma, in portal area and around the central vein was observed to increase significantly in MSG group compared to the control group, and melatonin tratment reversed this increase to the control levels.
Hepatic stellate cells differentiate into active myofibroblast like cells expressing a-SMA, in the presence of liver injury [44]. Long term activation of stellate cells causes fibrosis. Studies have shown that undifferentiated fetal hepatic stellate cells are of mesodermal origin and this finding was supported by their a-SMA expression [45]. A-SMA is an actin isoform and is not found in other liver cells except smooth muscle cells around the vessels [46]. VEGF expression in the liver of chronic hepatitis C carrier patients was observed to have a positive correlation with a-SMA expression and the degree of fibrosis. In a study in which stellate cells were evaluated by a-SMA immunohistochemistry, there was paralellism between cell activation degree and fibrosis level [47]. Another study using thioacetamide in animals reported that quiescent hepatic stellate cells expressed vimentin and desmin but not a-SMA, and that a-SMA immunoreactive cells were seen only along fibrotic lesions [48].
It was shown in mice that phagocytosis of apoptotic bodies derived from hepatocytes by stellate cells induced production of collagen 1, TGF-β1, and NADPH Oxidase (NOX) dependent superoxide [49]. NOX-2 activation and stellate cell activation were reported to be directly related and it was also reported that there was a central enzyme in ROS mediated collagen increase. Melatonin treatment in acute liver ischemia-reperfusion injury provides amelioration by inhibiting inflammation, oxidative stress, and apoptosis [50]. In the present study, a few pale stained a-SMA and NOX-2immunoreactive cells in control group were increased in number and intensely stained in MSG group. The number of reactive cells and staining intensity decreased after melatonin treatment. These findings are in line with the above mentioned previous studies.
NF-κB is a transcription factor that plays a role in a number of pathological processes related to inflammation, cell injury and death, as well as immune reactions [51]. Whike pro-inflammatory cytokines such as IL-6 and TNF-a increase due to inflammation as a result of oxidative stress caused by MSG, the expression of NF-κB is also greatly increased [52]. Recent studies have shown that MSG causes systemic injury through oxidative stress [5,6]. Activation of TNF-receptor 1 signal and cell death by oxidative stress induced by MSG was linked to DNA damage caused by NF-κB activation, as well as to the relation between oxidative stress and TNF-a [52]. Anti inflammatory effect of melatonin has been proven by many studies. It was determined that pro-inflammatory cytokines such as TNF-a and IL-1β secreted from Kupffer cells decreased and NF-κB expression was inhibited after melatonin treatment in rats with liver fibrosis induced by CCL4 [53]. Also, antiinflammatory and ameliorating effects of melatonin on fulminant hepatitis in rabbits caused by rabbit hemorrhagic disease virüs were reported [54]. In our study, NF-κB immunoreactive cells were observed to increase in liver tissues of MSG group and decreased in melatonin treated group.
Transforming Growth Factor β (TGF-β) provides cell growth and has a function in formation of extracellular matrix [55]. Kupffer cells secrete TGF-β and PDGF, which constitute a potent mitogenic factor for hepatic stellate cells [56]. In addition, IL-1 and TNF-a expression in Kupffer cells lead to activation of the NF-kB signaling pathway, which promotes the survival of hepatic stellate cells. TGF-β binds to its receptor and then substrates of the receptor, Smad2 and Smad3, are phosphorylated [57]. Smad6 and Smad7, which are responsible for negative regulation of TGF-β, are responsible for autoinhibition of activity. In studies in which various plant extracts were used as therapeutic agents, it was determined that protection against liver fibrogenesis could be achieved by inhibiting Smad 2 signaling pathway and by increasing Smad7 activity [58,59]. In a study in which liver fibrosis was created by bile duct ligation, it was found that collagen synthesis and a-SMA expression decreasedad-nd Smad7 expression increased [60]. It has been shown in previous studies that melatonin, which has been proven to have anti-inflammatory and fibrosis preventing effects, exerts these effects through inhibition of TGF-β1/Smad signaling pathway [61]. In another study, it was observed that oxidative stress occurred in the liver as a result of CCl4 administration and the hepatic fibrosis process began [44]. TGF-β1 and Smad2/3 immunoreactivity were increased and Smad7 levels were decreased in the injury group. It was determined that fibrosis was prevented and amelioration was achieved in the group given melatonin. In our study, a remarkable increase was observed in TGF-β1 and Smad2 immunoreactive cells in MSG group compared to the control group. Immunopositive cells were decreased in melatonin treated group compared to the MSG group. Smad7 immunohistochemistry results showed that a significant decrease was observed in the percentage of immunoreactive cells in the MSG group compared to the control and MSG+melatonin groups.
Conclusion
In the present study, it was observed that melatonin supplementation in liver damage caused by MSG consumption contributed to the reversal of histopathological and inflammation findings, and profibrotic parameters to normal levels. In order to better understand the pathophysiology of the damage caused by MSG and the mechanisms of protective effects of melatonin, we suggest that further molecular and functional studies should be conducted.
Author Statements
Declaration of Conflicting Interests
The authors declare that the research was conducted in the absence of any potential conflict of interest.
References
- Bera TK, Sk K, Yadav P, Mukherjee P, Yadav S, et al. Effects of monosodium glutamate (MSG) on human health: a systematic review. World J Pharmaceut Sci. 2017; 5: 139-144.
- Kusumler AS, Özgün AD. Gida katki maddelerinin saglik üzerine etkileri, Saglik ve Yasam Bilimleri Dergisi 2020; 2: 22-26.
- Rogers PJ, Blundell JE. Umami and appetite: effects of monosodium glutamate on hunger and food intake in human subjects. Physiol Behav. 1990; 48: 801-804.
- Abdul-Hamid M, Galaly SR, Ahmed RR, Hamdalla HM. Monosodium glutamate as a food additive: toxic implications and the protective role of quercetin. Merit Res J Med Med Sci. 2018; 5: 1-19.
- Onyema OO, Farombi EO, Emerole GO, Ukoha AI, Onyeze GO. Effect of vitamin E on monosodium glutamate induced hepatotoxicity and oxidative stress in rats, Indian J Biochem Biophys. 2006; 43: 20-24.
- Y Nakanishi, K Tsuneyama, M Fujimoto, TL Salunga, K Nomoto, et al. Monosodium glutamate (MSG): a villain and promoter of liver inflammation and dysplasia. J Autoimmun. 2008; 30: 42-50.
- El-Kenawy AE-M, Osman HE, Daghestani MH. The effect of vitamin C administration on monosodium glutamate induced liver injury: an experimental study. Exp Toxicol Pathol. 2013; 65: 513-21.
- Gobatto CA, Mello MAR, Souza CT, Ribeiro IA. The monosodium glutamate (MSG) obese rat as a model for the study of exercise in obesity. Res Commun Mol Pathol Pharmacol. 2002; 111: 89-101.
- Niijima A, Togiyama T, Adachi A. Cephalic-phase insulin release induced by taste stimulus of monosodium glutamate (umami taste). Physiol Behav. 1990; 48: 905-908.
- Gaspar RS, Benevides ROA, Fontelles JLL, Vale CC, Franca LM, et al. Reproductive alterations in hyperinsulinemic but normoandrogenic MSG obese female rats. J Endocrinol. 2016; 229: 61-72.
- Othman SI, Bin-Jumah M. Histomorphological changes in mono-sodium glutamate induced hepato-renal toxicity in mice. Int J Pharmacol. 2019; 15: 449-456.
- Brzezinski A. Melatonin in humans. N Engl J Med. 1997; 336: 186-195.
- Yildirim A, Arabaci Tamer S, Sahin D, Bagriacik F, Kahraman MM, et al. The effects of antibiotics and melatonin on hepato-intestinal inflammation and gut microbial dysbiosis induced by a short-term high-fat diet consumption in rats. Br J Nutr. 2019; 122: 841-855.
- Caglikulekci M, Bilgin O, Canbaz H, Dirlik M, Bagdatoglu O, et al. Deneysel hepatik iskemi reperfüzyon modelinde melatonin uygulamasinin kan ve karaciger doku lipid peroksidasyonuna etkisi. Ulusal Cerrahi Dergisi 2006; 22: 93-98.
- P Boonnate, S Waraasawapati, W Hipkaeo, S Pethlert, A Sharma, et al. Monosodium glutamate dietary consumption decreases pancreatic β-Cell mass in adult Wistar rats. PloS One 2015; 10: e0131595-e0131595.
- Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010; 23: 270-299.
- Atici AE, Arabaci Tamer S, Levent HN, Peker Eyuboglu I, Ercan F, et al. Neuropeptide W attenuates oxidative multi-organ injury in rats induced with intra-abdominal sepsis. Inflammation. 2022; 45: 279-296.
- Acikel Elmas M, Atay N, Bingol Ozakpinar O, Arbak S, Kolgazi M, et al. Morphological evaluation of the effects of exercise on high-fat-diet-induced liver damage in rats. Turk J Gastroenterol. 2020; 31: 626-632.
- K He, L Zhao, ML Daviglus, AR Dyer, L Van Horn, et al. INTERMAP Cooperative Research Group. Association of monosodium glutamate intake with overweight in Chinese adults: the INTERMAP study. Obesity (Silver Spring). 2008; 16: 1875-1880.
- Yu T, Zhao Y, Shi W, Ma R, Yu L. Effects of maternal oral administration of monosodium glutamate at a late stage of pregnancy on developing mouse fetal brain. Brain Res. 1997; 747: 195-206.
- Prunet-Marcassus B, Desbazeille M, Bros A, Louche K, Delagrange P, et al. Melatonin reduces body weight gain in Sprague Dawley rats with diet-induced obesity. Endocrinology. 2003; 144: 5347-5352.
- Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010; 51: 679-689.
- Stacchiotti A, Grossi I, García-Gómez R, Patel GA, Salvi A, et al. Melatonin effects on non-alcoholic fatty liver disease are related to microRNA-34a-5p/Sirt1 axis and autophagy. Cells. 2019; 8: 1053.
- Tsai CC, Lin YJ, Yu HR, Sheen JM, Tain YL, et al. Melatonin alleviates liver steatosis induced by prenatal dexamethasone exposure and postnatal high-fat diet. Exp Ther Med. 2018; 16: 917-924.
- Tawfik MS, Al-Badr N. Adverse effects of monosodium glutamate on liver and kidney functions in adult rats and potential protective effect of vitamins C and E. Food Nutr Sci. 2012; 3: 651-659.
- Farombi EO, Onyema OO. Monosodium glutamate-induced oxidative damage and genotoxicity in the rat: modulatory role of vitamin C, vitamin E and quercetin. Hum Exp Toxicol. 2006; 25: 251-259.
- Ogeturk M, Kus I, Kavakli A, Zararsiz I, Ilhan N. Effects of melatonin on carbon tetrachloride-induced changes in rat serum. J Physiol Biochem. 2004; 60: 205-210.
- Hatzis G, Ziakas P, Kavantzas N, Triantafyllou A, Sigalas P, et al. Melatonin attenuates high fat diet-induced fatty liver disease in rats. World J Hepatol. 2013; 5: 160-169.
- Eweka A, Igbigbi P, Ucheya R. Histochemical studies of the effects of monosodium glutamate on the liver of adult wistar rats. Ann Med Health Sci Res. 2011; 1: 21-29.
- Atef H, El-Morsi DA, El-Shafey M, El-Sherbiny M, El-Kattawy HA, et al. Monosodium glutamate-induced hepatotoxicity and oxidative stress: pathophysiological, biochemical and electron microscopic study. Med. J. Cairo Univ. 2019; 87: 397-406.
- Bhattacharya T, Bhakta A, Ghosh SK. Long term effect of monosodium glutamate in liver of albino mice after neo-natal exposure. Nepal Med Coll J. 2011; 13: 11-16.
- Elbassuoni EA, Ragy MM, Ahmed SM. Evidence of the protective effect of l-arginine and vitamin D against monosodium glutamate-induced liver and kidney dysfunction in rats. Biomed Pharmacother. 2018; 108: 799-808.
- Miskowiak B, Kesa B, Limanowski A, Partyka M, Filipiak B. Long-term effect of neonatal monosodium glutamate (MSG) treatment on reproductive system of the female rat. Folia Morphol (Warsz). 1999; 58: 105-113.
- Ibrahim MA, Khalifa AM, Saleh AA, Tammam HG. Histopathological and histochemical assessment of monosodium glutamate-induced hepatic toxicity and the amelioration with propolis. Ain Shams J. Forensic. Med. Clin. Toxicol. 2019; 33: 24-36.
- Aktas C, Kanter M, Erboga M, Mete R, Oran M. Melatonin attenuates oxidative stress, liver damage and hepatocyte apoptosis after bile-duct ligation in rats. Toxicol Ind Health. 2014; 30: 835-844.
- Zhang JJ, Meng X, Li Y, Zhou Y, Xu DP, et al. Effects of melatonin on liver injuries and diseases. Int J Mol Sci. 2017; 18: 673.
- Malik VBT, Ahluwalia P. Studies on effect of monosodium glutamate (MSG) on various fractions of lipids and certain carbohydrate metabolic enzymes in liver and blood of adult male mice. Toxicol Lett. 1994; 74: 69-77.
- Wells RG. Cellular sources of extracellular matrix in hepatic fibrosis. Clin Liver Dis. 2008; 12: 759-768.
- Mak KM, Leo MA, Lieber CS. Alcoholic liver injury in baboons: transformation of lipocytes to transitional cells. Gastroenterology. 1984; 87: 188-200.
- Hu W, Ma Z, Jiang S, Fan C, Deng C, et al. Melatonin: the dawning of a treatment for fibrosis? J Pineal Res. 2016; 60: 121-131.
- Tahan V, Ozaras R, Canbakan B, Uzun H, Aydin S. Melatonin reduces dimethylnitrosamine-induced liver fibrosis in rats. J Pineal Res. 2004; 37: 78-84.
- Kang JW, Hong JM, Lee SM. Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis. Journal of Pineal Research. 2016; 60: 383-393.
- Yu-Rong W, Hong R-T, Xie Y-Y, Xu J-M. Melatonin ameliorates liver fibrosis induced by carbon tetrachloride in rats via inhibiting TGF-β1/Smad signaling pathway. Curr Med Sci. 2018; 38: 236-244.
- Lotersztajn S, Julien B, Teixeira-Clerc F, Grenard P, Mallat A. Hepatic fibrosis: Molecular mechanisms and drug targets. Annu Rev Pharmacol Toxicol. 2004; 45: 605-628.
- Tsuchida T, Friedman S. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017; 14: 397-411.
- Elzamly S, Agina HA, Elbalshy AE-L, Abuhashim M, Saad E, et al. Integration of VEGF and a-SMA expression improves the prediction accuracy of fibrosis in chronic hepatitis C liver biopsy. Appl Immunohistochem Mol Morphol. 2017; 25: 261-270.
- Washington K, Wright K, Shyr Y, Hunter EB, Olson S, et al. Hepatic stellate cell activation in nonalcoholic steatohepatitis and fatty liver. Hum Pathol. 2000; 31: 822-828.
- Tennakoon AH, Izawa T, Wijesundera KK, Golbar HM, Tanaka M, et al. Characterization of glial fibrillary acidic protein (GFAP)-expressing hepatic stellate cells and myofibroblasts in thioacetamide (TAA)-induced rat liver injury. Exp Toxicol Pathol. 2013; 65: 1159-1171.
- Jiang JX, Venugopal S, Serizawa N, Chen X, Scott F, et al. Reduced nicotinamide adenine dinucleotide phosphate oxidase 2 plays a key role in stellate cell activation and liver fibrogenesis in vivo. Gastroenterology. 2010; 139: 1375-1384.
- Sun C-K, Chen C-H, Chang C-L, Chiang H-J, Sung P-H, et al. Melatonin treatment enhances therapeutic effects of exosomes against acute liver ischemia-reperfusion injury. Am J Transl Res. 2017; 9: 1543-1560.
- Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. 2005; 7: 395-403.
- Banerjee A, Mukherjee S, Maji BK. Efficacy of Coccinia grandis against monosodium glutamate induced hepato-cardiac anomalies by inhibiting NF-kB and caspase 3 mediated signalling in rat model. Hum Exp Toxicol. 2021; 40: 1825-1851.
- Wang H, Wei W, Wang N-P, Gui S-Y, Wu L, et al. Melatonin ameliorates carbon tetrachloride-induced hepatic fibrogenesis in rats via inhibition of oxidative stress. Life Sci. 2005; 77: 1902-1915.
- Laliena A, Miguel BS, Crespo I, Alvarez M, González-Gallego J, et al. Melatonin attenuates inflammation and promotes regeneration in rabbits with fulminant hepatitis of viral origin. J Pineal Res. 2012; 53: 270-278.
- I. Güven. Insan karaciger hücre hattinda (Hepg2) Tgf-β/ Smad yolaginin arastirilmasi. Erciyes üniversitesi Saglik Bilimleri Enstitüsü, Tibbi Biyokimya, Master of Science Thesis, Kayseri, Turkey, 2016.
- Henriques MSM, Leite JA, M Diniz MFF, Araújo MST. Immunohistochemistry pattern of hepatic inflammatory and insulin resistance markers in experimental model of nonalcoholic steatohepatitis. J Bras Patol Med Lab. 2014; 50.
- Dewidar B, Meyer C, Dooley S, Meindl-Beinker N. TGF-β in hepatic stellate cell activation and liver fibrogenesis-updated 2019. Cells. 2019; 8: 1419.
- Jiang Y, Wang C, Li Y-Y, Wang X-C, An J-D, et al. Mistletoe alkaloid fractions alleviates carbon tetrachloride-induced liver fibrosis through inhibition of hepatic stellate cell activation via TGF-β/Smad interference. J Ethnopharmacol. 2014; 158: 230-238.
- Huang W, Li L, Tian X, Yan J, Yang X, et al. Astragalus and Paeoniae Radix Rubra extract (APE) inhibits hepatic stellate cell activation by modulating transforming growth factor-β/Smad pathway. Mol Med Rep. 2015; 11: 2569-2577.
- Dooley S, Hamzavi J, Breitkopf K, Wiercinska E, Said HM, et al. Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology. 2003; 125: 178-191.
- Kim J-Y, Park J-H, Kim K, Leem J, Park K-K. Melatonin inhibits transforming growth factor-β1-induced epithelial-mesenchymal transition in AML12 hepatocytes. Biology (Basel). 2019; 8: 84.