
Special Article: Brain Scan
Austin J Neurol Disord Epilepsy. 2024; 10(1): 1052.
Massive Multiple Sclerosis Reactivation in A 72-Year-Old Man After Natalizumab Discontinuation: A Case Report
Riccardo Nistri¹*; Antonio Ianniello¹; Ilaria Tomasso²; Lanfranco De Carolis²; Carlo Pozzilli¹
1Department of Human Neuroscience, La Sapienza University, Rome, Italy
2Ospedale Sant’Andrea, Università la Sapienza, Roma, Italy
*Corresponding author: Riccardo NistriDepartment of Human Neuroscience, La Sapienza University, Rome, Italy Email: riccardo.nistri16@gmail.com
Received: January 17, 2024 Accepted: February 23, 2024 Published: March 01, 2024
Abstract
Natalizumab is a monoclonal antibody with excellent efficacy in controlling multiple sclerosis activity. However, its discontinuation can lead to a massive inflammatory reactivation. So far, the decision to discontinue or continue disease-modifying therapy in older patients remains a matter of debate and the final choice is based on several clinical features including age, time since last relapse, number of previous therapies, and disease duration.
In this report, we describe a massive multiple sclerosis reactivation in a 72-year-old patient with diabetes and hypertension who had to stop natalizumab due to a high JCV index after 12 years of treatment, during which clinical and radiological stability were maintained. This severe relapse resulted in a widespread high activity on brain MRI and an EDSS worsening.
This case confirms that the management of natalizumab discontinuation is challenging, even in older patients. Factors such as age and comorbidities can further complicate the choice of alternative treatments.
Introduction
Multiple Sclerosis (MS) is the leading cause of non-traumatic disability among young adults [1]. So far, more than nineteen Disease-Modifying Treatments (DMTs) have been approved for the treatment of MS, each characterized by distinct mechanisms, safety profiles, and tolerability [2]. Natalizumab (NTZ) is a monoclonal antibody that selectively blocks the alfa-4 subunit of integrins, thereby inhibiting the passage of leukocytes across the blood-brain barrier. Despite its great efficacy in controlling MS activity, NTZ discontinuation represents a real challenge for physicians, as it could lead to a massive inflammatory reactivation known as rebound [3]. The most common cause of NTZ discontinuation is the John Cunningham virus (JCV) positivity, which can lead to severe and often fatal encephalitis known as Progressive Multifocal Leukoencephalitis (PML) [4]. JCV is a ubiquitous virus whose seroconversion increases with age, being detected in 60-80% of the population at the age of seventy. The higher prevalence of JCV among older individuals, coupled with the natural reduction of MS activity with age reduces NTZ usage in older patients. Whether age plays a protective role in mitigating rebound after NTZ discontinuation has still to be elucidated.
Case
A 72-year-old patient diagnosed with insulin-dependent diabetes and hypertension at the age of 50, presented with his first neurological symptom in 2001, characterized by a left-eye optic neuritis, which was successfully treated with intravenous steroid. An MRI showed multiple periventricular lesions without enhancement, while no lesions were detected in the spinal cord. A strict neurological follow up ensued until February 2005, when the patient received the diagnosis of MS following the onset of hypoesthesia in the left hemiface. A new periventricular lesion and a lesion in the medulla oblongata were observed on MRI, prompting the initiation of treatment with Interferon beta 1b (Betaferon) three times a week. In September 2008, he developed hypoesthesia and weakness in both arms and his left leg, accompanied by severe hyperglycemia (384 mg/dl). Increasing insulin doses led to an improvement of the neurological symptoms. Nevertheless, he also received a three-day course of intravenous steroids (500mg). In June 2011, the annual MRI showed the presence of 6 new encephalic lesions and a new spinal cord lesion. Consequently, Betaferon was discontinued and a new therapy with NTZ was started. Under NTZ treatment the patient enjoyed 12 years of clinical and radiological stability, maintaining a stable EDSS score of 3.5.
In April 2015, after 36 infusions, a Stratify test routinely conducted for NTZ surveillance regimen, yielded a positive result with an index of 1.97. Due to the PML risk, the patient began a strict radiological follow-up with new MRI scans every 3-6 months. The infusion schedule gradually transitioned from a 4-week regimen to a 6-week one. The patient continued NTZ treatment for an additional 7 years, until October 2022, when, due to advanced age and the high PML risk, with persistent JCV titer between 1.90 and 2.10, along with a total number of 95 infusions, the decision was made to discontinue NTZ. After 5 months, in March 2023, the patient developed gait ataxia. He underwent a new brain and spinal cord MRI which revealed ten new lesions with contrast enhancement (Figure 1). For this reason, a five-day steroid treatment was initiated, resulting in a partial recovery. A further brain and spinal cord MRI performed one month later (April 2023) showed a complete radiological stability.
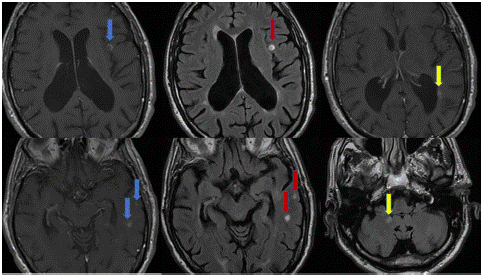
Figure 1: In the left column T1 images with new Gad enhancing lesions (blue arrows) and the corresponding FLAIR images in the center (red arrows); in the right column two more enhancing lesions (yellow arrows).
Discussion
The treatment of elderly patients with MS has become an increasingly important topic in the everyday practice. Particularly, therapy discontinuation after the age of 55 years is a subject of debate as showed by the recent DISCOMS study [5]. So far, the decision to discontinue or continue a DMT remains a medical controversy. Physicians’ choices primarily rely on parameters such as age, MRI stability, time since last relapse, number of previous DMTs used and disease duration. However, there is no certainty of preventing new relapses after discontinuation. Additionally, DMTs are associated with age-related adverse events [6], further complicating treatment decisions in this category of patients.
NTZ discontinuation represents a real clinical challenge, as the risk of worsening after this event has been demonstrated to be one in three, with even higher risks for patients with EDSS scores above 3.0 [7].
Our case showed a severe reactivation in a 72-year-old patient, due to a rebound determined by NTZ discontinuation. This is an important example, highlighting that rebound can occur irrespective of age. Therefore, initiating a new DMT should be always considered after NTZ discontinuation, in order to avoid this well-known complication, that does not appear to be influenced by age. This observation in an elderly patient is aligned with the findings of Chappuis et al [8] in middle-aged patients, where NTZ discontinuation is associated with a greater risk of inflammatory activity compared to other DMTs, prompting the need to start a new therapy.
Comorbidities are another important factor to consider, particularly when there are three or more, as they have been associated with a higher relapse rate [9]. Diabetes and hypertension, both diagnosed in our patient, have also been associated with greater disability progression and the presence of contrast-enhancing lesions on brain MRI [10]. At the same time, these conditions are associated with vascular events that can mimic an MS relapse, adding complexity to the clinical picture.
Moreover, as JCV seroconversion increases over time, other patients successfully treated with NTZ for many years, could need a drug switch for safety reasons. While the transition to another monoclonal antibody is straightforward for young adults, the high risk of DMT adverse events in older patients can limit possible available choices.
Nevertheless, evaluating a switch to a DMT with a favorable safety profile and minimal impact on the immune system, might provide a practical compromise between MS treatment and age-related comorbidities.
Conclusions
NTZ rebound is a complication that can occur even in older patients. Considering another DMT, with a better safety profile, is advisable for older patients, in order to prevent MS reactivation.
References
- Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, et al. Multiple sclerosis. Nat Rev Dis Primers. 2018; 4: 43.
- Melamed E, Lee MW. Multiple Sclerosis and Cancer: The Ying-Yang Effect of Disease Modifying Therapies. Front Immunol. 2019; 10: 2954.
- Borriello G, Prosperini L, Mancinelli C, Giannì C, Fubelli F, Pozzilli C. Pulse monthly steroids during an elective interruption of natalizumab: a post-marketing study. Eur J Neurol. 2012; 19: 783–7.
- Cortese I, Reich DS, Nath A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat Rev Neurol. 2021; 17: 37–51.
- Corboy JR, Fox RJ, Kister I, Cutter GR, Morgan CJ, Seale R, et al. Risk of new disease activity in patients with multiple sclerosis who continue or discontinue disease-modifying therapies (DISCOMS): a multicentre, randomised, single-blind, phase 4, non-inferiority trial. Lancet Neurol. 2023; 22: 568–77.
- Prosperini L, Haggiag S, Tortorella C, Galgani S, Gasperini C. Age-related adverse events of disease-modifying treatments for multiple sclerosis: A meta-regression. Mult Scler. 2021; 27: 1391–402.
- Prosperini L, Annovazzi P, Capobianco M, Capra R, Buttari F, Gasperini C, et al. Natalizumab discontinuation in patients with multiple sclerosis: Profiling risk and benefits at therapeutic crossroads. Mult Scler. 2015; 21: 1713–22.
- Chappuis M, Rousseau C, Bajeux E, Wiertlewski S, Laplaud D, Le Page E, et al. Discontinuation of second- versus first-line disease-modifying treatment in middle-aged patients with multiple sclerosis. J Neurol. 2023; 270: 413–22.
- Kowalec K, McKay KA, Patten SB, Fisk JD, Evans C, Tremlett H, et al. Comorbidity increases the risk of relapse in multiple sclerosis: A prospective study. Neurology. 2017; 89: 2455–61.
- Weinstock-Guttman B, Zivadinov R, Mahfooz N, Carl E, Drake A, Schneider J, et al. Serum lipid profiles are associated with disability and MRI outcomes in multiple sclerosis. J Neuroinflammation. 2011; 8: 127.