
Review Article
Austin J Nanomed Nanotechnol. 2023; 11(1): 1069.
Biosynthesis of Selected Nanoparticles for Biomedical Applications
Javier Christian Ramirez-Perez*
Institute of Physics, Nuclear Physics Department, University of Sao Paulo, Sao Paulo, Brazil
*Corresponding author: Javier Christian Ramirez-Perez Institute of Physics, Nuclear Physics Department, University of Sao Paulo, Sao Paulo, Brazil. Tel: +51(11)982916169 Email: jramire7@kent.edu
Received: August 02, 2023 Accepted: August 22, 2023 Published: August 29, 2023
Abstract
Nanoparticles (NPs) are materials with a size scale of 100 nm or less. They are produced using a combination of chemical and physical techniques, and frequently include the use of hazardous substances. These hazardous substances are difficult to remove from nanoparticle surfaces, making them bioincompatible and limiting their application as biomedical nanoparticles. Nanoparticles can currently be synthesized biologically using methods based on green chemistry. The fusion of nanotechnology and biology has given rise to a new field called nanobiotechnology, which aims to harness biological entities such as actinomycetes, algae, bacteria, fungi, viruses, yeast, and plants in a number of physicochemical, biochemical, and biophysical processes. Plant extracts have been used in biological applications because they are a rich source of phytomolecules and because their capping and reducing capabilities have been used in the production of metal and metal oxide NPs. The biosynthesized NPs have not only been examined for their antibacterial, antifungal, antioxidant, anticancer, and biocompatibility properties but also for their morphology using a number of analytical techniques. This approach, which is affordable and environmentally friendly, could substitute traditional chemical and physical methods for biomedical applications.
Keywords Biosynthesis; Nanoparticles; Plant extracts; Biomedical application; Properties
Introduction
Nanotechnology is one of the most significant and fast growing fields involved in the synthesis of Nanoparticles (NPs) with at least one dimension in the size range of 1–100 nm resulting in high surface to volume ratio [65], this is a very important unique property that allows them to be used in different fields of chemical, food, electronic, and medical industries [61,62,64]. Synthesizing NPs can be done in a variety of ways, including physical, chemical, and biological methods (Figure 1). Physical and chemical approaches are commonly used in the commercial synthesis of NPs. Physical (laser ablation, arc discharging, radiation, photolithography, ball milling, etc.), chemical (sol-gel, solvothermal, co-precipitation, pyrolysis, chemical redox reaction, micro-emulsion, thermal decomposition, co-precipitation, sonochemical, flow-injection, hydrolysis/thermolysis of precursors, sol–gel and electrospray synthesis, photosynthesis, and chemical vapor deposition produces hazardous byproducts by utilizing harsh reducing agents, organic solvents, and poisonous compounds [1,8,56,61] and biological (plants, fungi, bacteria, algae, virus, yeast, plants, leaves, fruits, etc.) methods [14,46,62]. Physical and chemical methods often involve the use of harsh synthetic chemicals as reducing and capping agents in the chemical method, such as citrate, sodium borohydride, hypophosphite, and hydrazine [18,46], results in harsh chemicals adsorbing on the surface of produced NPs, increasing their toxicity (Vijay and Anu, 2017). Physical processes, on the other hand, such as plasma, pulsed laser, gamma radiation, and mechanical milling, demand a lot of energy and take longer to scale up than green synthesis [7,15,48]. As a result, the use of hazardous chemicals and solvents, as well as the demand for high energy, the reduction of capping agents, and scaling issues, are all key issues in the synthesis of these NPs. These toxic chemicals utilized in the synthesis of the NPs remain on the surface of the NPs even after several washing cycles. This compromises the biocompatibility of the NPs, and hence limits the applications of the NPs in particular in biomedicine, which could have a harmful impact on the environment and human being. In fact, the presence of residual hazardous chemical species on the surface of the synthesized NPs cannot be removed easily and could prohibit their biological and clinical applications [30].
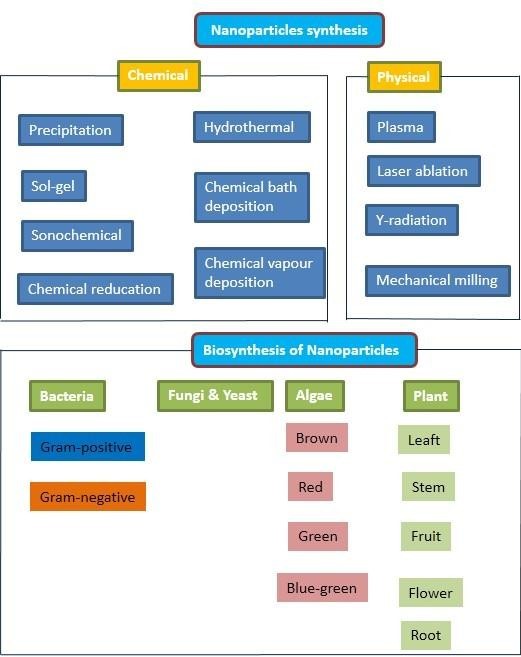
Figure 1: Common methods for synthesizing nanoparticles.
Among the various biological methods to synthesize the NPs using microorganisms, such a bacteria is facilitated by their ability to precipitate those molecules out of cells, as those nanoparticles can be obtained using cell filtration which is considered beneficial for intracellular synthesis [30]. It has been known that bacteria synthesize nanoparticles in two ways, one through extracellular mechanisms and the second within cells. In this regard, the fi rst to synthesize nanoparticles gold (AuNP) from the extracellular wall of B. subtilis using the gold chloride solution. In another work, Seudomonas stutzeri AG259 was used to produce AuNP nanoparticles accumulated inside cells, characterized by being of a small size (1–200 nm) using the NADH dependent enzyme (Zhao et al., 2020). Also Pseudomonas aeruginosa bacteria was reported to have the capacity to biogenic distinct types of nanoparticles within cells such as nanoparticles Pd, Ag, Rh, Ni, Fe, Co, Pt, and Li without ever having to use any type of stabilizing agent and electron, then studies continued to test many bacteria in their ability to synthesize nanoparticles such as those conducted on Escherichia coli, B.subtilis, B.megaterium, B.cereus, Pseudomonas aeruginosa, Klebsiella pneumoniae, Alteromonas and Ochrobactrum sp.etc. [6,7,36]. Recently a group of bacteria has developed itself to become more resistant to antibiotics, making it more dangerous for the human race include hazardous and toxic chemicals like sodium borohydride and N, N-dimethyl formamide along with culture media [19,26,43,48] these NPs have been used in various health and industry products, which could be toxic for health and environment [1,22,25]. Now the nanoparticles are a newly innovative new agent with a wider surface area relative to their small size and with physical and chemical properties that make them highly capable of dealing with these pathogenic bacterial species and that can penetrate the cell wall [49]. Biosynthesis of silver nanoparticles was reported by using the marine brown alga Padina pavonia and fungi Aspergillus niger and their applications in biochemical sensors, electronic equipment stimulants, biopsy, tumor imaging, drug making and pharmaceutical preparation methods [4]. However, Biosynthesis of NPs employing bacteria, fungi, virus, yeast, algae, and other organisms necessitates a series of complex procedures, including microbial cell culture maintenance, extensive incubation times, several purification steps, energy consumption, and cost-effectiveness. The use of different plant extracts as a source of reducing and capping agents in the biosynthesis of NPs is an alternative to the traditional ways for solving these challenges and minimizing environmental concerns. Green chemistry has attracted scientists' interest due to its environmentally favorable attributes. The usage of plants is considered more effective, easily scalable, and economical than other biological methods [19,43]. Biosynthesis of nanoparticles using plant extracts methods is an emerging field that has several advantages because it occurs at a relatively ambient temperature and pressure, take a little time than those other methods of one-post synthesis being robust and environmentally friendly [45,52]. Therefore, Biosynthesis of NPs using plant extracts has gained tremendous attention over the last five years as an alternative, because it uses natural resources and is believed to be more biocompatible and worthwhile approach over the chemical, physical and some biological techniques. In the next section is described examples of biosynthesis of metal and metal oxides nanoparticles reported during this time for biomedical applications.
Plant Extract Used in the Biosynthesis of Nanoparticles
Plant’s based biological methods do not require toxic chemical substances as they used plant’s phytomolecules (phenolic compounds and vitamins, polyphenols, alkaloids, terpenoids, saponins, etc.) as reducing and capping agents for the nanoparticle’s synthesis [19]. Different plants and their other parts, such as stem, leaves, flowers, seeds, shoots, etc., have been widely utilized to synthesize several kinds of nanoparticles (Saranya et al., 2008). The biosynthesis of NPs using plant extracts is considered as more significant and cost-effective technique with special concern on environment and ecosystem conservation approach. It is safe, simple, energy efficient and ecofriendly as compared with many toxic synthesis methods [11,34]. The concept of NPs biosynthesis was first used Β-D-glucose, which served as a strong reducing and stabilizing agent for the silver NPs. The biosynthesis of Nanoparticles (NPs) with various sizes, shapes, chemical compositions, magnetic, electrical, and optical properties offers new promise to the biomedical area. Metals (Au, Ag, Pt, Pd, Mn, Zn, Cu, etc.) and metal oxide (Au, Ag, Pt, Pd, Mn, Zn, Cu, etc.) The biosynthesis of NPs has gained significant momentum due to their remarkable properties (high dispersion, small size, and high surface to volume ratio) compared with those of their macroscopic counterparts, and NPs have widely used and studied for antibacterial, antimycotic, antibiofilm, antioxidant, and anticancer applications in nanomedicine [50]. Although numerous metals exist in nature, only a few of them such as Fe, Ag, Au, Sn, and Pt were synthesized extensively in nanostructured form. Different plants and their other parts, such as stem, leaves, flowers, seeds, shoots, etc., have been widely utilized to synthesize several kinds of nanoparticles. Some of these plants are biologically active but have serious biocompatibility issues, and some are not biologically active. For example, many researchers have used Stevia leaf extract for different nanoparticle synthesis. But in 2019, leaves and crude extracts of this plant were included in an FDA import alert with concerns about their safety for use in foods or supplements and potential for toxicity [20,34]. Therefore, nanoparticles for biomedical applications must be needed to be synthesized with such plants that have no biocompatible issue, and they should also be biologically active. In this regard, Abutilon indicum has significant importance as this plant is biologically active and has no biocompatibility issues [45]. Abutilon indicum is a medicinal plant and extensively utilized for the treatment of different types of diseases, including blood dysentery, allergy, bronchitis, pulmonary tuberculosis, mumps, rheumatism, diarrhea, vaginal infections, cleaning wounds and ulcers, relieving thirst, piles, jaundice, urinary disease, and leprosy since ancient times [40,45]. Abutilon indicum has numerous natural products, including flavonoids, phenolic acids, alkaloids, saponins, terpenoids, etc., that enable this plant to show different biological activities (analgesic, anti- inflammatory, antimicrobial, antidiabetic, anticancer, antioxidant, hepatoprotective, anti-asthmatic, antilarvicidal, etc.) [41].
Plants are rich in a wide variety of secondary metabolites that can be used to produce nanoparticles, which have advantages such as energy efficiency, cost effectiveness, and environmental stewardship [5]. Most significantly, biosynthesized NPs have good medicinal properties such as considerable antimicrobial, antioxidant, anti-inflammatory, anti-diabetic and anti- cancerous potential compared to NPs synthesized by other traditional methods [5,14,53]. Due to these advanced properties, biosynthesized NPs are gaining attention in the field of medicine, pharmaceutical, agriculture, plant protection and food processing science [1,23,58]. The advanced effect of AgNPs in the medical field, for example, was attributed to the sustained release of nanoparticles from aggregates that act as AgNP carriers. AgNPs produced from plant extracts with sizes ranging from 5 to 100 nm have exhibited promising biological properties. These researchers used a variety of methodologies and varying conditions (pH, temperature, etc.) to achieve greater AgNPs for antibacterial, anti-parasitic, antioxidant, antidiabetic, and anticancer applications. It will be examined at some recent examples of metal and metal oxide NPs that have been published in the literature in the next section. Raman spectroscopy is useful in detecting vibrational modes of molecules and can be used to identify vibrational signals of a variety of chemical species that are attached to the surface of nanoparticles during synthesis [46,53,58]. For example, using Surface-Enhanced Raman Scattering (SERS) it was possible to measure single molecular attachments on Ag nanoparticles [45,46].
Characterization of Nanoparticles Using Physicochemical Techniques
Nanoparticles are highly dependent on their size, shape, and surface structure and processing tends to introduce surface imperfections. These surface imperfections can signifi cantly impact on the overall nanoparticle surface physicochemical properties. The resulting nanoparticles are characterized using various techniques to determine properties such as particle size, size distribution, crystallinity, shape, and surface area and stability. This is of particular importance if the properties of nanoparticles need to be homogeneous for a particular application. Properties of nanoparticles need to be homogeneous for a particular application. In the case of biosynthesis of nanoparticles, the aqueous metal ion precursors from metal salts are reduced and as a result, a colour change occurs in the reaction mixture. This is the fi rst qualitative indication that nanoparticles are being formed. One technique that can be used to detect the presence of nanoparticles in solution. After the reaction, nanoparticles can be separated from the colloid by high speed centrifugation and then examined using advanced nanocharacterization techniques [45,46].
UV-visible and Color
The characterization of nanoparticles begins with a visual color change which works on the principle of Surface Plasmon Resonance (SPR). The color change occurs when the size of the particles increases, in the case of gold it is from deep red to purple. The varying color changes are due to LSPR that they exhibit, and it lies in the visible region of the electromagnetic spectrum, which means that particular portion of the wavelength in the visible region is absorbed while another portion gets refl ected and the emitted wavelength will refl ect its own color. The absorbance of these color changes is measured using UV-Visible spectroscopy [12]. The characteristic optical property exhibited by the metallic nanoparticles is due to the oscillations of the conduction band of electrons surfacing the nanoparticles. For example, AuNPs synthesized using C. inerme produced SPR values in the range of 400–700 nm The peaks also demonstrate the stability of the nanoparticles which increases with time [16]. A UV-Visible spectrophotometer was used to detect the absorption maxima of plant leaf extract and phytomolecules-coated NiO nanoparticles, for example. The UV- Visible spectra are shown in Figure 2A-B. The results show that Abutilon indicum leaves extract has a broad UV-Visible absorption spectrum (200 to 385 nm), indicating the presence of flavonoids and polyphenolic chemicals (Figure 2A). The presence of hydroxyl (O-H) functional groups in these phytomolecules causes them to absorb UV radiation, according to reports [4,25]. Phytomolecules-coated NiONPs, on the other hand, showed two distinct absorption bands. NiO was found to have an absorption peak at 330 nm, while phytomolecules were found to have an absorption peak at 380 nm (Figure 2B).
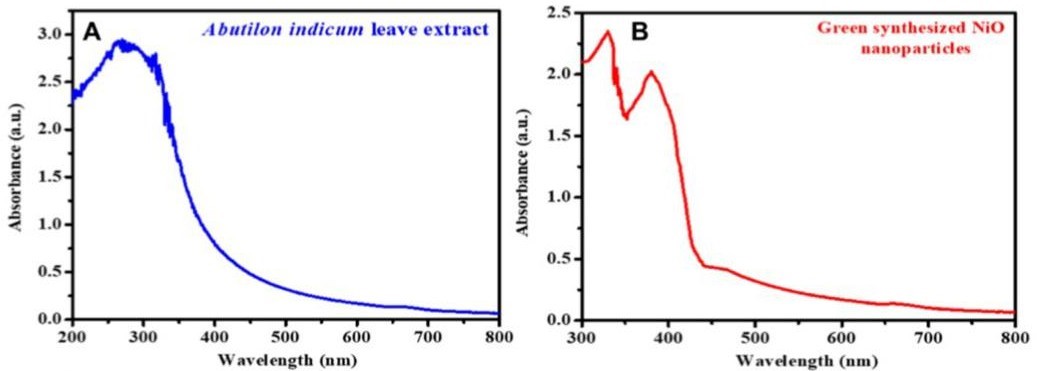
Figure 2: UV-Visible spectra of (A) Abutilon indicum leaves extract, and (B) phytomolecules-coated NiO nanoparticles [25].
DLS and ZP
DLS (Dynamic Light Scattering) – also known as photon correlation spectroscopy – and ZP (Zeta Potential) have developed as easy table-top techniques for investigating the (hydrodynamic) size and surface charge of NPs, respectively, in conventional lab conditions. The ZP, or electrokinetic potential, is the potential at the slipping/shear plane of a colloidal particle moving in an electric field [2,13,46]. The work required to transport a unit positive charge from infinity to the surface without acceleration is defined as the electric potential of a surface. During electrophoresis, the particle with adsorbed EDL moves towards the electrodes (in this case, the positive electrode), with the sliding plane acting as an interface between the mobile particles and the dispersion. At this slipping plane, the ZP denotes the electrokinetic potential. At the slipping plane, the ZP represents the potential difference between the EDL (electric double layer) of electrophoretically mobile particles and the layer of dispersant surrounding them. DLS spectroscopy can be used to determine size distribution and quantify the surface charge of nanoparticles suspended in a liquid. For example, DLS size distribution intensity was used to confirm the stability and the dispersity of biosynthesized Palladium Nanoparticles (PdNPs) made from the Coroupita guianensis Aubl. (cannonball fruits) [13], the hydrodynamic diameter of PdNPs was obtained as 27 nm (Figure 3a). In addition, the stability of PdNPs was analyzed through zeta potential and mobility of nanoparticles in colloidal suspension. Figure 3b shows the zeta potential of PdNPs measured as -17.7 (mV) indicating that the largest negative potential value could be due to capping agent, which generates repulsive forces between the NPs in growth medium. The presence of a positive or negative zeta potential indicates outstanding physical colloidal stability [3]. The electrostatic repulsions between the particles are the reason for this. In another method, combining the particles could reveal a lower-magnitude Zeta potential.
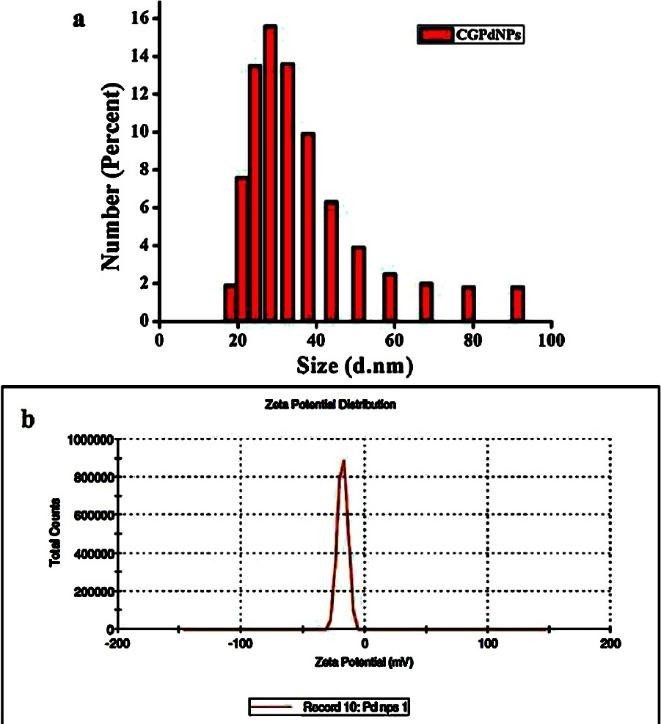
Figure 3: (a) DLS analysis of PdNPs biosynthesized using fruit extract of C. guianensis Aubl. and (b) Zeta potential measurements of PdNPs [13].
EDX and XRD
EDX (energy-dispersive X-ray spectroscopy) is a technique used for the chemical characterization or elemental analysis of any given sample. It can be used to basically determine the number of nanoparticles produced with a thin fi lm of bacterial biomass [9]. Energy- Dispersive Spectroscopy (EDS) is used to determine the elemental composition of nanoparticles. Some studies also use more precise analysis using Selected Area Energy Dispersion (SAED) patterns to inspect how biosynthesized nanoparticles are [11]. The crystalline nature of gold nanoparticles was established using X-Ray Diffraction (XRD) analysis.
The sample preparation involved the reduced the gold nanoparticles solution to be capped on a glass surface and it has been one on the equipment which was effective at the voltage of 40 kv, and running at the voltage of 20 mA with Cu Ka radiations [17,24,42]. The majority of the crystalline nature of the nanoparticles using plant extracts has been analyzed with the XRD diffraction pattern and the mean size of these nanoparticles was calculated using Debye-Scherer equation [6]. XRD examination produces a diffraction pattern that is subsequently compared with data contained in a standard crystallographic database to determine structural information. Analysis of the XRD data identifi es crystallite size, structure, preferred crystal orientation, and phases present in samples [42]. The chemical composition of biosynthesized Au NPs using Viola betonicifolia leaves extract was investigated using Energy- Dispersive X-ray (EDX) and X-Ray Diffraction (XRD) spectroscopy to determine the crystallinity of the Au NPs generated. Figure 4A displays an XRD pattern that distinguishes peaks ascribed to the crystal planes of Au NPs (111), (200), (220), (311), and (222) [28]. Furthermore, the strength of the peaks in the XRD pattern indicates that the Au NPs are highly crystalline. TEM pictures of VB-Au NPs are shown in Figure 4B. The biosynthesized Au NPs are spherical with homogenous dispersity as seen by the TEM picture. Figure 4C shows that the particle size of the produced Au NPs was determined to be 13 nm using DLS. The chemical composition of the Au NPs is revealed by EDX analysis (Figure 4D). The EDX spectra reveal four distinct peaks that correspond to carbon, oxygen, nitrogen, and gold. Aside from gold, the EDX peaks (C, O, and N) were attributed to secondary metabolites adsorption on the surface of Au NPs from Viola betonicifolia leaves extract. As a result of these characterization findings, the successful production of VB-Au NPs utilizing a Viola betonicifolia leaf extract was confirmed.
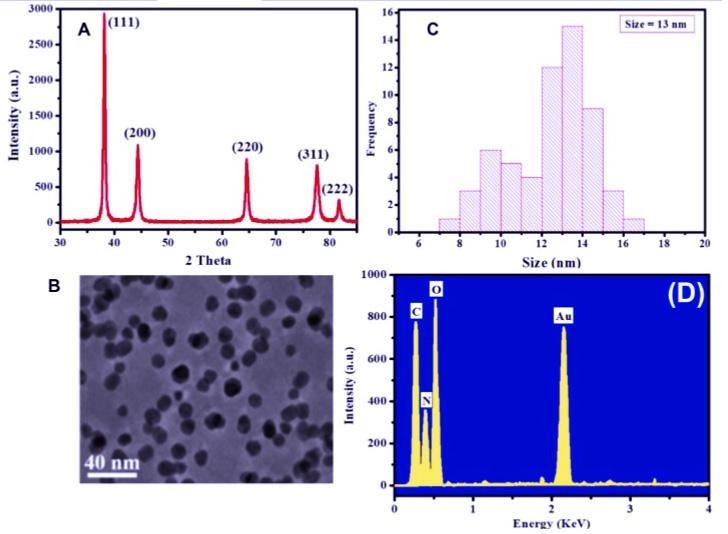
Figure 4: (A) XRD pattern, (B) TEM image, (C) size distribution, and (D) EDX pattern for the green biosynthesized AuNPs [58].
SEM, TEM and FESEM
The analytical tools of Scanning Electron Microscopy (SEM), Tunneling Electron Microscopy (TEM), particle analyzers, and fi eld Emission Scanning Electron Microscopy (FESEM) were used to analyze the size and morphology of the metal/metal oxide NPs. Some studies also use more precise analysis using High Resolution Transmission Electron Microscopy (HRTEM) to characterize the size and morphology of nanoparticles. The analysis from Scanning Electron Microscope (SEM) requires sample preparation, which includes the formation of thin fi lms of carbon coating on copper grids. These fi lms were prepared by dropping a minute amount of sample onto the grip while the remaining solution was removed with. blotting paper, followed by further drying under the mercury lamp for a minimum of 5 min only (Gnanasekar et al., 2018; Dumas et al., 2015). The SEM images demonstrate different shapes of the nanoparticles biosynthetized using plant extracts have been found to be in various shapes like the rectangle, square, cubic and mono or poly-disperse among an average diameter (Ramirez-Perez et al., 2021). Some studies also use more precise analysis using Field Emission Scanning Electron Microscope (FESEM) to characterize the size and morphology of nanoparticles.
The sample preparation for Transmission Electron Microscopy (TEM) characterization involves placing a drop of solution on a carbon- coated copper grip which is dried at a room temperature, while the residual solution is removed with the blotting paper. The TEM gives information on the morphology and shapes of these nanoparticles [45]. As pointed out the characterization of nanoparticles using TEM depicted the shape and average size of nanoparticles. While the SEM pattern confi rms the crystalline nature of the nanoparticles [46]. Biosynthesized Cu/CuO NPs from Syzygium alternifolium plant extract, for example, were characterized using TEM and SEM (Figure 5a-b) [56]. To evaluate the structure of the nanoparticles, FESEM analysis was used to characterize the biosynthesis of ZnO NPs utilizing Saussurea lappa plant root (rhizome) extract. Figure 6a-b show a hexagonal wurtzite structure with hexagonal form of produced ZnO NPs.

Figure 5: Characterization of biosynthesized copper/copper oxide nanoparticles (Cu/CuO NPs). (a) TEM image of CuO NPs synthesized from Syzygium alternifolium (b) SEM image [35].
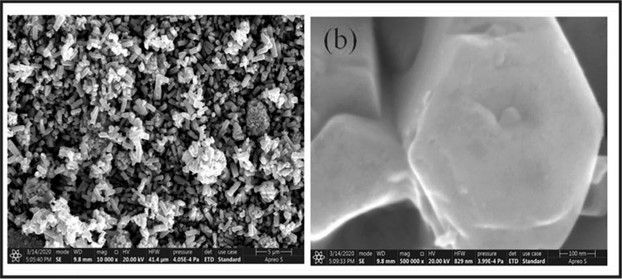
Figure 6: FESEM images of biosynthesized ZnO nanoparticles from Saussurea lappa plant extract (a) at 5 μm and (b) at 100 nm [28].
FTIR and Raman Spectroscopy
Fourier Transform Infrared (FTIR) spectroscopy provide unique, label free tool for studying biomolecular composition and dynamics without perturbing the sample. FTIR can be used to investigate surface chemistry and identify surface residues such as functional groups like carbonyls and hydroxyls moieties that attach to the surface during nanoparticle synthesis. The vibrational characteristics of all the functional organic groups present in the plant extracts including metabolites, which can be liable for reducing and capping agents leading to profi cient stabilization of the nanoparticles [46]. The nanoparticle-rich suspension is dried fully and crushed with KBr pellets before being analyzed for composition. 512 scans are verified to achieve adequate results in order to obtain a good signal/noise ratio [47]. Raman spectroscopy is useful in detecting vibrational modes of molecules and can be used to identify vibrational signals of a variety of chemical species that are attached to the surface of nanoparticles during synthesis. For example, using Surface-Enhanced Raman Scattering (SERS) it was possible to measure single molecular attachments on nanoparticles [47]. Raman spectroscopy is looks to be more adapted to studying biological systems, where several of the target biomolecules, such as metabolites compounds are present at low concentrations, the method is rather unselective with respect to the numerous molecules present in the biological milleu of capping nanoparticles, given very congested spectra [46,47]. FTIR analysis was used to identify functional groups of phytomolecules that act as reducing and capping agents to synthesize phytomolecule-coated NiO nanoparticles. The FTIR spectra of Abutilon indicum leaves extract and phytomolecules-coated NiO nanoparticles are shown in Figure 7. The FTIR spectrum data of Abutilon indicum leaves extract displayed a number of distinct peaks at 3410 cm-1 (OH), 2990 cm-1 (C-H), 1702 cm-1 (C=O), 1650 cm-1 (amide I and amide II), 1630 cm-1 (C=C), 1259 cm-1, (O-H), 1140 cm-1 (C-O). Furthermore, the FTIR spectra of the phytomolecules-coated NiO nanoparticles revealed not only the peaks corresponding to Ni-O at 430 cm-1, but also many FTIR signals related to the phytomolecules of Abutilon indicum leaves extract. Similar FTIR signals for Ni-O were also reported by Khan et al., 2021. The effective synthesis of phytomolecules-coated NiO nanoparticles capped with bioactive molecules plant phytochemicals was thus confirmed by the FTIR data.
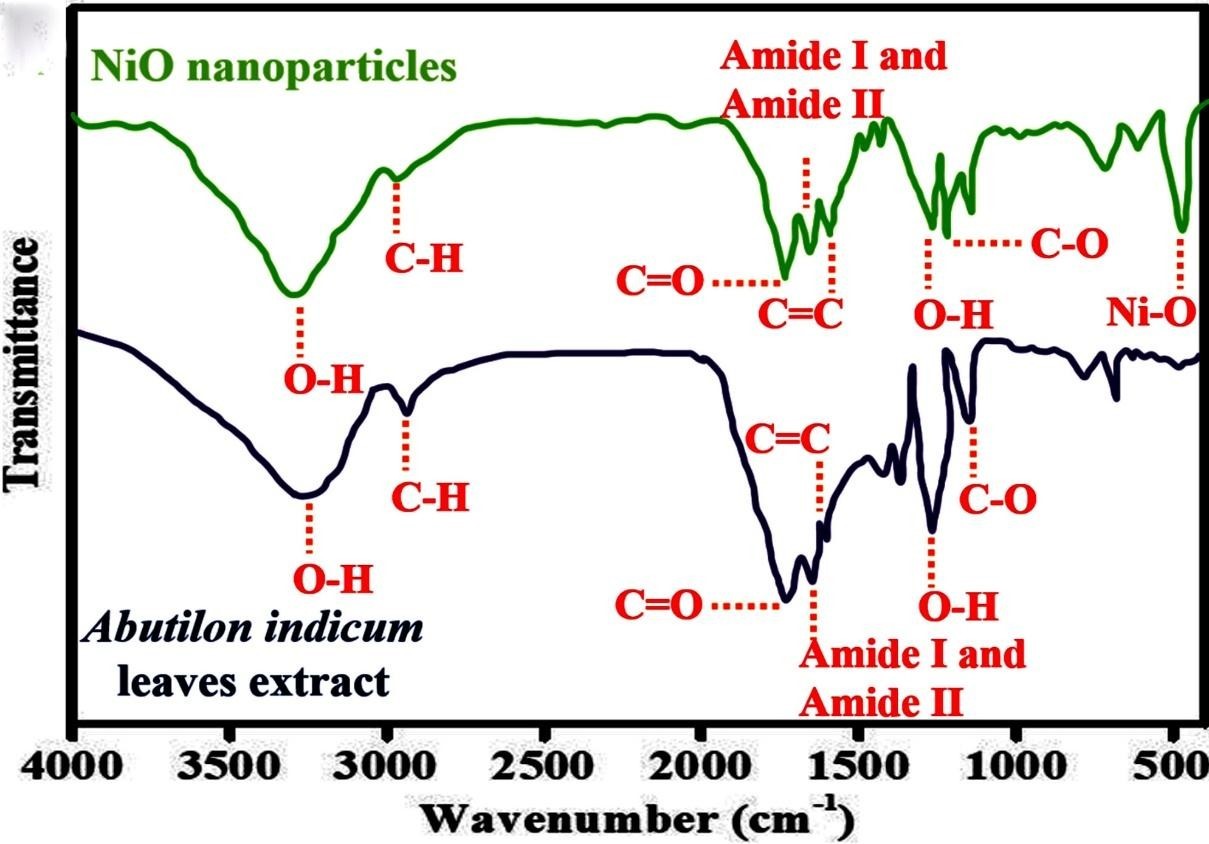
Figure 7: FTIR spectrum of Abutilon indicum leaves extract, and hytomolecules- coated NiO nanoparticles [37].
Characterization of Nanoparticles for Biomedical Applications
In the present day, nanoparticles are often utilized as catalysts in cosmetics, textiles, photonics, agriculture, and heavy industries. There have been a slew of studies published on the use of plant-based metal/metal oxide in medicine. According to available evidence, metal and metal oxide NPs synthesized with plant extracts are primarily applied as antibacterial, antifungal, and anticancer agents. The details, including the mechanism, will be discussed in the following sections.
Antibacterial
Studies have revealed that metal/metal oxide NPs inhibit the growth of Gram- negative and Gram-positive bacteria [A]. Plant-mediated metal/metal oxide NPs effectively suppressed Gram-negative bacteria such as E. coli, Enterococcus sp., Proteus sp., and Klebsiella sp. in urinary tract infection. This suggests that these NPs could be used to treat infections like these. Plant-derived NPs can also suppress K. pneumonia, which can be hazardous if it spreads to other parts of the body [7]. Gram-positive bacteria including Staphylococcus aureus, Bacillus subtilis, and Streptococcus pyrogenes, for example, have been shown to be inhibited by Cu and CuO NPs. Respiratory and skin infections are caused by S. aureus, while diarrhea is caused by B. subtilis. S. pyrogenes, on the other hand, infects people and causes illnesses such as rheumatic fever and scarlet fever [11]. As illustrated in Figure 8A-C. Au NPs created utilizing plant extract Viola bentonicifolia showed exceptional antibacterial inhibitory performance when compared to plant leaves extract and chemically generated AuNPs. The mechanism of antibacterial action for AuNPs made using this method has been described. Microbial strains have been found to be killed by oxidative stress caused by intracellular Reactive Oxygen Species (ROS) production [58]. Metal nanoparticles interact with bacteria to form Reactive Oxygen Species (ROS), which can cause oxidative stress in the cell, resulting in the destruction of organelles and biomolecules. The improved antibacterial action of AuNPs synthesized with Viola betonicifolia plant extracts, for example, could be attributed to a synergistic effect of the nanoparticle's physical properties and the adsorption of biologically active phytomolecules from Viola betonicifolia leaves extract on.
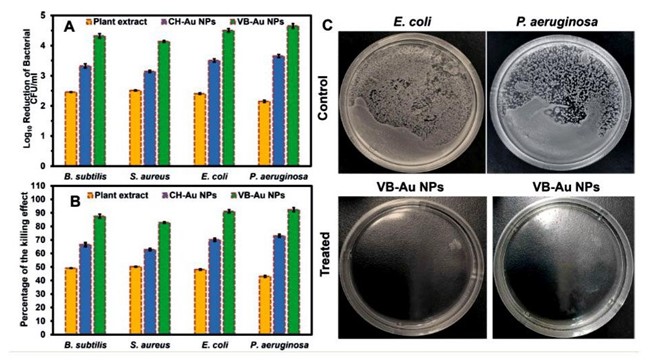
Figure 8: Antibacterial activity of VB-Au NPs in terms of (A) log10 reduction (p<0.0004) and (B) % killing efficiency of bacterial strains in comparison to leaves extract of Viola betonicifolia and CH-Au NPs (p<0.0004). (C) Representative images of control and treated E. coli and P. aeruginosa with VB-Au NPs [58].
Antifungal
People can become infected after coming into direct touch with fungus. Aspergillius flavus, a pathogenic fungus, causes liver cancer in people when they eat bad food. Inhaling A. fumigates airborne spores can cause long-term lung disease. After combining biosynthesized AgNPs and antibiotics, their potential was investigated. When compared to the antibiotic and biosynthesized their surface. The results also revealed that the AuNPs produced were more effective against Gram-negative than Gram-positive bacteriological species. This could be due to structural and compositional differences in Gram-positive and Gram-negative bacteria's cell walls (Figure 9). Gram-positive bacteria have a thick layer of peptidoglycan that teichuronic and teichoic acids are covalently bonded to. Gram-negative bacteria, on the other hand, have a thin peptidoglycan coating on the outside and a negatively charged lipopolysaccharide coating [44,49,54,60]. Produced AuNPs appear to have a larger inhibitory effect on Gram-negative bacteria than on Gram-positive bacteria as a result of these considerations. Bindhu et al. (2014) discovered the same higher inhibitory action of gold NPs produced using diverse plants.

Figure 9: Comparison of Gram-negative and Gram-positive bacteria cell wall and proposed antibacterial mechanism of green synthesized VB-Au NPs. Created with BioRender.com [58].
AgNPs alone, combinations had higher antibacterial activity against all strains tested. Antibiotics (Tetracycline, Bacitracin, Ciprofloxacin, and Cefixime) had no effect on Candida albicans. When AgNPs were introduced to the antibiotic discs and evaluated, they showed strong antifungal AgNPs were introduced to the antibiotic discs and evaluated, they showed strong antifungal activity, which was even greater than the biosynthesized AgNPs using C. albicans alone. Cu/CuO NPs were recently revealed to have antifungal properties, suggesting they could be employed to treat fungal infections [59]. According to a study, CuNPs produced from A. eriophyllum Boiss leaf extract were found to be more resistant to fungi like C. guilliermondii and C. krusei than bacteria [2]. Because the fungus's cell wall is made up of numerous layers of lipids, NPs have a hard time entering the organism [48]. A recent study found that the production of CuO NPs caused cell wall destruction and the accumulation of Reactive Oxygen Species (ROS) in A. flavus, demonstrating an antifungal impact [21]. CuO NPs may have antifungal effects through a number of pathways, according to the limited evidence (Figure 10).
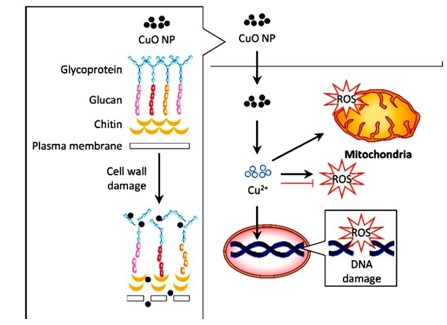
Figure 10: Graphical representation of the proposed mechanism for antifungal activity in response to copper oxide nanoparticles (CuO NPs). The CuO NPs distorted the cell wall of fungus such as Aspergillus fl avus. Internalization of these particles induced Reactive Oxygen Species (ROS) generation, which resulted in DNA and mitochondrial damage that contribute to the antifungal activity [35].
Anticancer
Plant-mediated metal/metal oxide NPs have been shown to have anticancer properties in numerous investigations, particularly in breast, colon, blood (leukemia) cancers (Vijay et al., 2017), cervical, skin (epithelioma) [48], gastric [15], liver [7], lung [21] and ovarian [54] cancers. The cancer cells were seeded in a 96-well plate prior to NP treatment to test the anticancer effect of metal/metal oxide NPs [25]. They were kept at 37°C with a constant supply of 5% carbon dioxide in their respective media. Various colorimetric tests are employed in the research of cancer cell toxicity, including: MTT (3-(4,5- dimethylthiazol-2-yl)2,5-diphenyl tetrazolium bromide), MTS (3-(4,5- dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H- tetrazolium), and WST (4-[3-(4-Iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzenesulfonate). Based on the reductase activity of the mitochondria, these tests quantify metabolic activity in live cells [60]. The toxicity of NPs to cancer cells varies vary greatly in size and structure. In A549 lung cancer cells and MCF-7 breast cancer cells, previous research have suggested that large- sized metal NPs have a higher half-maximal inhibitory concentration (IC50) than small-sized NPs [48]. Remarkably, studies have found that NPs larger than 200 nm have a lower IC50 on MCF-7 and MDA-MB-231 breast cancer cells than NPs smaller than 5–24 extract and standard anticancer drug. Results of cell viability percentage demonstrated that phytomolecules-coated NiONPs exhibited significant cytotoxic effect on HeLa cancer cells compared to chemically synthesize NiONPs (Figure 11). On HeLa cancer cells, the cytotoxic effect of Biosynthesized NiONPs was comparable to that of traditional anticancer medications, with both showing more than 50% cytotoxicity [44]. These metal/metal oxide NPs were made from a different plant source under different conditions. Rehana et al. (2017) conducted a cytotoxic investigation on various cancer cells (MCF-7, HeLa, Hep-2, and A549) treated with CuO NPs generated utilizing various types of plant extracts from Azadirachta indica, Hibiscus rosa-sinensis, Murraya koenigii, Moringa oleifera, and Tamarindus indica. Furthermore, NiO NPs biosynthetized using Abulition indicum were evaluated for anticancer potential using MTT assay against HeLa cancerous cells line compared to chemically synthesize NiO NPs and leaves extract of Abutilon indicum compared to a plant.
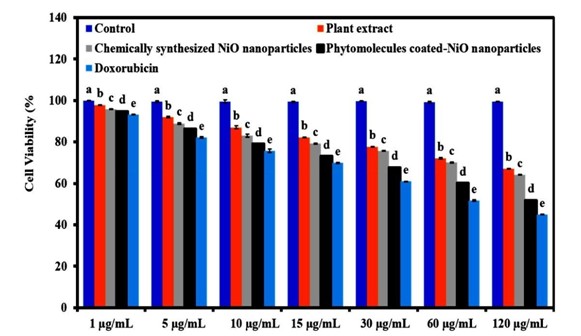
Figure 11: The anticancer potential of phytomolecules-coated NiO nanoparticles compared to leaves extract of Abutilon indicum, chemically synthesized NiO nanoparticles, and standard drug in terms of cell viability percentage against HeLa cancer cells (F=7225.663, p<0.001) [22]. Note: Tukey based heterogeneous lower-case letters represent significant pairs.
Biosynthesis of NPs using Plant Extracts
Holoptelea integrifolia - AgNPs
Holoptelea Integrifolia (HI) is well documented plant in Ayurvedic System, having multiple applications to cure diseases like arthritis, inflammation, anaemia, skin diseases, liver disorders etc. [29]. Researchers have noticed antioxidant, antibacterial, antimutagenic, antivenom and antitumor qualities of HI [55]. The medicinal activities depend on the chemical composition of HI. The isolated and documented compounds of HI are 1, 4- naphthalenedione, hexacosanol, stigmasterol, B-amyrin, betulin, betulinic acid, epifriedlin, octacosanol, friedlin, holoptelin-A and holoptelin-B. After detailed literature study. AgNPs have shown various biomedical applications including antimicrobial, anti-parasitic, antioxidant, antidiabetic and anticancer [29]. Recent study reported AgNPs biosynthesis using leaves extract of HI. 8 g of hade dried leaves were powdered put into 200 ml distilled water and heated at 60°C for 15 min, then cooled and the capping and reducing agents as OH and NH functional groups on the AgNPs surface were supernatant was filtered, light green extract as clear solution was obtained and stored at 4-10°C. Next 50 ml of silver nitrate solution (2 mM) was mixed with 100 ml of leaves extract. A colour change from light green to colloidal black indicated the formation of AgNPs. After 30 min, 100 ml of reaction mixture was centrifuged for 5 min. at 5000 rpm. The solid particles as sediment were collected, washed with distilled water followed by ethanol and further dried at room temperature. The dried material was characterized using UV-vis, FTIR, XRD and FESEM, EDX analysis. HI confirmed by FTIR, and demonstrated stable aggregates of spherical shapes of size 32-38 mm. In addition, nutrient and biomedicinal were reported, antioxidant activities [11,55], and 75% for the DPPH, metal chelating and NO assay; anti-diabetic (87%), anti-inflamatory (binding constant 2.6x10-4), and antibacterial (MIC from 75 to 150 uL) activities were found for biosynthesized AgNPs. The main results are summarized in Table 1.
Clerodendrum inerme – Au and AgNPs
Clerodendrum inerme (C. inerme) has been widely used for treating venereal infections, cough, fever, skin diseases, microbial infections, rheumatism, leprosy, etc., as the raw materials for preparing Au and AgNPs [27,30]. By reducing Ag+ and Au+3 using C. inerme extract, Ag and AuNPs capped with various functional groups were obtained (Rossolini et al., 2016) [36]. 10 g of dried and pawdered leaves of C. inerme was added to 100 mL of DI water and boiled for 5 min. then filtered to obtain a yellowish colored extract and stroed at 4°C. A total of, repectively, 1 mM of HAuCl4.3H2O and AgNO3 were added into 25 mL of leaves extract of C. inerme. The mixtures of gold and silver salts were both heated at 80°C for 65 min with continuous stirring to obtain a ruby red and a dark brown dispersion of CIAu and CI-Ag NPs, respectively. After centrifugation, the obtained CI-Au and CI-Ag NPs were washed with DI water and dried at 70oC [31]. Both samples were characterized by XRD, UV- vis, FTIR, TEM, EDX and DLS. The analyses reported both samples spherical in morphology and average sizes 5.82 (Cl-Au) and 5.54 (Cl-Ag), respectively. Absorption spectra of the leaf extract and the nanoparticles analyses revealed biomolecules such as polyphenols and flavonoids. FTIR spectra of Cl-Au NPs and ClAg-NPs the C.inerme leaves extracts showed the same aromatic compounds, this suggested that many of the organic functional units in the leaf´s extracts were left on the surface of Cl-Au NPs and ClAg-NPs. In the biosynthesis mechanism processes, metal ions are reduced to metal nanoparticles with only the leaves extract as reducing and capping reactants in the synthetic reaction [27].
During synthesis, gold and silver salts in leaves extract first dissociated into their ions, such as Au3+ and Ag+,then phytomolecules in C. inerme, such as flavonoids, phenolics, carbohydrates, cardiac glycosides, and anthraquinones can first reduce both metal ions into their zero-valent species [36,43]. While capping agents, such as terpenoids, tannins, saponins, alkaloids, and proteins, can encapsulate the Au and Ag zero-valent species to stabilize them. The biosynthesized NPs were evaluated for different biological activities such as antibacterial and antimycotic against different pathogenic microbes (B. subtilis, S. aureus, Klebsiella, and E. coli) and (A. niger, T. harzianum, and A. flavus), respectively, using agar well diffusion assays. The antimicrobial propensity of NPs further assessed by Reactive Oxygen Species (ROS) Glutathione (GSH) and FTIR analysis. Biofilm inhibition activity was also carried out using colorimetric assays. The antioxidant and cytotoxic potential of CI-Au and CI-Ag NPs was determined using DPPH (1,1 Diphenyl-2-Picryhydrazyl) free radical scavenging and MTT assay, respectively. The CI-Au and CIAg NPs were demonstrated to have much better antioxidant in terms of % DPPH Scavenging (75.85% and 78.87%), respectively. They exhibited excellent antibacterial, antimycotic, biofilm inhibition and cytotoxic performance against pathogenic microbes and MCF-7 cells compared to commercial Au and Ag NPs functionalized with dodecanethiol and PVP (polyvinylpirrolidone), respectively [27]. As shown in Table 1, the biocompatibility test confirmed that CI-Ag and CI-Au NPs are more biocompatible at concentrations of 1–50 M.
R. virgate FeO NPs
Rhamnus virgate (R. virgate), a large shrub/small tree armed with pointed spines, is widely distributed in Asia and South America [19]. The main chemicals found in R. virgata are physcion, emodin, kaempferol, rhamnocitrin, quercetin, herbacetin and maesopsin. R. virgata has shown strong therapeutic potentials, such as antioxidant, antimicrobial, laxative, effects, emetic, purgative properties mostly used in the treatment of parasitic infections, spleen affection and leg swelling [1,26]. Also, it is a rich source of alkaloids, flavonoids, anthraquinones and can function as an ideal candidate for the reduction of iron salt to nanoiron. Iron oxide (FeO)NPs have potentially gained the attention of the scientific community due to their unique properties and potential applications in biomedicine, diagnostics, therapeutics and materials engineering [22,25]. Currently, (FeO) NPs have been used in the controlled release of drugs in tissue repairing, diagnosis and treatment of solid tumors through magnetic resonance imaging. These diverse applications of FeONPs can be attributed to their remarkable properties, such as higher surface area, small band gap (2.1 eV) and stability [1,23,25]. Biosynthesis of NPs employing plant extract may be influenced by phytomolecules present in plant extract; flavonoids, phenols, antioxidants and physicochemical factors governing the kinetics of areaction [49]. Recently have been reported the use of leaves extract of R. virgata for the biosynthesis of (FeO) NPs. 10 g of dried leaves, were powdered and mixed with 250 ml of DI water and filtered the extract, in 50 ml of filtered R. virgata extract, 3 g iron acetate salt was dissolved at 60°C for 2 hr with stirring after cooling, the solution was centrifuged 10 min and filtered. The obtained powder, assumed as FeO NPs, was further calcinated at 500°C to achieve pure phase crystalline FeONPs and characterized using UV-vis, XRD, SEM, TEM, FTIR, Raman spectroscopy and FTIR. The results found a mean crystallite size of 20 nm and spherical shape of FeO NPs [32]. Detailed in vitro biological activities revealed significant therapeutic potentials for IONPs. Potential antibacterial and antifungal activities are reported for (FeO) NPs. Bioinspired (FeO) NPs have shown potential results against cancerigen cHepG2 nontumorigenic cells, the minimal concentration required for 50% inhibition in vitro (IC50:13.47 μg/ml). Dose dependent cytotoxicity assays were revealed against Leishmania tropica (KMH23 strain) promastigotes (IC50: 8.1 μg/ml) and amastigotes (IC50: 20.8 μg/ml) using different concentrations of (FeO) NPs (1-200 μg/ml). The cytotoxic activity was also studied using brine shrimps, and their IC50 value was calculated as 32.41 μg/ml. Significant total antioxidant capacity was achieved (TAC 51.4%), and (DPPH scavenging, 79.4%) and total reducing power (TRP 62%). The biocompatibility assays using red blood cells (>200 μg/ml) and macrophages (>200 μg/ml) confirmed the biosafe nature of (FeO) NPs (Table 1).
Abutilon indicum - NiO NPs
Many reports state that different parts (leaves, roots, fruits, and flower) of Abutilon indicum (A. indicum) is a rich source of several biologically active phytochemical constituents, including phenolics, tannins, starch, glycosides, flavonoids, alkaloids, steroids, and carbohydrates compounds [4]. Due to the presence of these natural products, A. indicum has been extensively used for treating numerous types of ailments (blood dysentery, allergy, bronchitis, pulmonary tuberculosis, mumps, rheumatism, diarrhea, vaginal infections, cleaning wounds, and ulcers, relieving thirst, piles, jaundice, urinary disease, etc.) in traditional medicine [19,43]. In one study by GC-MS, Saranya et al (2016) have reported the triamcinolone acetonide, hydroxybenzoic acid ester, arabinitol pentaacetate, 5-thio-D-glucose, abutilin A, Z-11-hexadecenoic acid, 3- hydroxy-beta-ionol, 10- methoxydihydrocorynantheol, 10-methoxycorynan-17- ol,etc. in the leaves extract of Abutilon indicum. Moreover, Kuo et al (2008) have isolated the thirty compounds (Β-sitosterol and stigmasterol, vanillin, methylcoumarate, 4-hydroxyacetophenone, p-hydroxybenzaldehyde, aurantiamide acetate, methyl indole-3-carboxylate, 3,7-dihydroxychromen-2-one, methylparaben, scoparone, scopoletin, syringaldehyde, 1-methoxycarbonyl-Β- carboline, 4-hydroxy-3-methoxy-trans-cinnamic acid methyl ester, trans-p- coumaric acid, thymine, adenine, methyl 4-hydroxyphenylacetate, riboflavin, 1- lycoperodine, 3-hydroxy-Β-damascone, adenosine, p-hydroxybenzoic acid, 3-hydroxy-Β-ionol, N-feruloyl tyrosine, vanillicacid, and benzoic acid) from the extract of Abutilon indicum [20,50]. Hence, due to these phytomolecules, leaves extract of A. indicum can be performed role as reducing and capping agents. 20 g of A. indicum leaves powder was mixed with 120 mL of methanol and 180 mL of distilled water at 50oC, stirring, and filtered. 150 mL of leaf extract mixed with 3 g of Ni(NO3)26H2O, after, 1.5 mL of ethylene glycol was added into the reaction mixture and stirred continuously at 30oC. Separately, 150 mL leaves extact was mixed with 3 g citric acid, stirred at 30oC. After, both solutions were mixed, and the resultant reaction mixture obtained the greenish color dispersion of NiO NPs. After filtration, the obtained phytomolecules-coated NiO NPs were washed and dehydrated and calcinated at 500oC for 120 min. Finally the black colo phytomolecules-coated NiO NPs were obtained for characterization and biomedical tests [30]. The nickel salt first ionizes into its respective ions (Ni+2) in the presence of leaves extract, according to the likely molecular pathway for the biosynthesis of NiO NPs utilizing A. indicum. As demonstrated in Figure 12, phytomolecules (starch, glycosides, flavonoids, carbohydrates, polyphenols, and so on) of leaves extract efficiently reduce nickel metallic ions to metallic oxide nanoparticles (NiO) via redox reaction. Additionally, once NiO nanoparticles are produced, phytomolecules (tannins, alkaloids, steroids, proteins, and so on) stabilize and capped them. NiO NPs were characterized using XRD, SEM, EDX, DLS, UV-vis, FTIR. The average size of NiO NPs was 446.5 nm. In addition, biomedical applications including, antibacterial activity, cytotoxic activity, HeLa cancer cell viability, antioxidant activity, and biocompatibility evaluation. The phytomolecules-coated NiO nanoparticles synthesized using plant’s leaves extract showed excellent antibacterial performance by inhibiting the growth of Gram-negative and Gram- positive, e.g. bacterial strains E. coli, B. bronchiseptica, B. subtilis, and S. aureus) by presenting highest zone of inhibitions (18±0.58 mm, 21±0.45 mm, 22±0.32 mm, and 23±0.77 mm) [30]. They demonstrated comparable antibacterial activity compared to the standard antibiotic drug. The phytomolecules-coated NiONPs were further exhibited significant anticancer activity against the HeLa cancer cell lines, and HeLa cancer cells by exhibiting the least cell viability percentage (51.74±0.35%) compared to plant extract which was also comparable to the standard anticancer drug (Doxorubicin). The phytomolecules-coated NiONPs were also demonstrated excellent antioxidant activity, (79.87±0.43% DPPH inhibition) and biocompatibility (>90% cell viability) with fibroblast cells, Table 1 gives an overview of the findings. It was interesting to note that A. indicum leaves extract was also found active towards antibacterial, antioxidant, and anticancer activities. Thus, antioxidant, antibacterial, and anticancer activities results displayed the potential of phytomolecules-coated NiONPs for their biomedical applications. These results further recommended that phytomolecules-coated NiONPs exhibited excellent biological activities and biocompatibility due to the synergetic effect (physical properties and adsorbed phytomolecules on their surfaces).

Figure 12: The probable mechanistic synthesis pathway for the fabrication of phytomolecules-coated NiO nanoparticles using Abutilon indicum leaves extract. Notes: Adapted from Khan et al., 2020) [27].
Viola betonicifolia-MnO2 NPs
Leaves extract of Viola betonicifolia (L.), a species of family Violaceace (V) is naturally available in several countries over the globe [45]. This plant has been widely exploited as purgative, astringent, diaphoretic, anticancer, antipyretic, and employed for treating various diseases, such as nervous disorders, epilepsy, cough, skin disorders, blood disorders, sinusitis, pharyngitis kidney diseases, bronchitis, and pneumonia [5,40,41]. In addition, the leaves extract of V. betonicifolia is a rich source of several biogenic phytomolecules, including alkaloids, flavonoids, tannins, phenolic compounds, saponins, and triterpenoids [14,53]. Also, V. betonicifolia is a rich source of numerous secondary metabolites, such as alkaloids, flavonoids, tannins, phenolic compounds, saponins, triterpenoids, and so on, that are biologically active towards different potential biomedical applications (Lu et al., 2021). Recently, V. betonicifolia was used for bioconversion of manganese ions to NPs, using leaves extract of Viola betonicifolia as a reducing and capping agent, for bioconversion of manganese ions to NPs (MnO2-NPs). Moreover, it is considered that biogenic plant phytomolecules may enhance their intrinsic properties, such as antioxidant, antibacterial, and anticancer compared to extracts [1,27,58]. Recently was reported the biosynthesis using leaves extract of V. betonicifolia, 20 g of dried leaves mixed with 150 ml of DI water and stirred at 60°C for 60 min to obtain leaves extract of V. betonicifolia and then filtered. The filtrate was collected and stored at 4°C. The biosynthesis of MnO2 NPs, 1 Mm of Manganese acetate was added to 25 ml of leaves extract of V. betonicifolia, the resulting mixture was heated at 40°C for 70 min with stirring at pH 7.15. The formation of MnO2NPs was visually tracked by observing the color change caused by the addition of a precursor to leaf extract. The reaction mixture’s color shift from yellowish green to brownish indicated the formation of the MnO2NPs. This color shift occurred as a consequence of the nanoparticle’s surface plasmon resonance action. The MnO2 NPs synthesized were separated by centrifugation from the reaction mixture, and were washed, dried and further calcined at 200°C for 3 h. Finally, the MnO2 NPs were characterized by XRD, EDX, TEM, DLS. The average size of MnO2NPs revealed 20 nm of spherical shape [6]. The antibacterial results reported that the Biosynthesized MnO2NPs displayed 4.14±0.03 and 4.65±0.07 log10 reductions in CFU of K. pneumoniae and S. aureus, respectively, with more than 80% killing efficiency. Oxidative stress caused by intracellular ROS production has been shown to destroy microbial strains [38]. Moreover, they also exhibited significant antioxidant potential, which was comparable to the external standard (ascorbic acid). Metal Nanoparticles (NPs) interact with bacteria to produce ROS, which can lead to oxidative stress inside the cell and the destruction of organelles and biomolecules.
No intracellular ROS species were produced in either bacterial cell under control, bacteria treated with MnO2NPs produced ROS comparable to that produced by H2O2. The antifungal results indicated that the synthesized MnO2NPs significantly reduced the CFU of A. flavus, T. harzianum, and A. fumigatus by 4.05±0.06, 4.32±0.07, and 4.63±0.05 log10 reductions, respectively, with a killing efficiency of more than 82 percent. The biofilm inhibition activity of MnO2NPs was evaluated against infectious bacterial and mycological species. The maximum inhibitory effects on MCF-7 melanoma cells were observed when all samples were concentrated to 120 μg/ml [6] (Table 1).
The enhanced antioxidant, antibacterial, antifungal, and biofilm inhibition efficacy might be attributed to the synergistic effect of the MnO2NPs’ physical properties and the adsorbed biologically active phytomolecules from the leaves extract of V. betonicifolia on their surface.
Viola betonicifolia-Au NPs
As aforementioned, plant leaf extracts have been utilized as reducing and capping agents in the production of NPs. It has been shown that phytochemical components of plants, such as alkaloids, polyphenols, flavonoids, and terpenoids, promote metal ion reduction and the subsequent production of metal NPs. Furthermore, it is believed that biogenic phytomolecules may enhance the intrinsic properties of nanoparticles, such as antioxidant, antibacterial, and anticancer activities, via their absorption onto the nanoparticle surface [10,12,29,55]. Among all noble metal nanoparticles, AuNPs have received considerable interest in recent years due to their, tunable Surface Plasmon Resonance (SPR) and unique optical and electrical characteristics and their potential applications in the fields of chronic disease [13,16,22,41]. V. betomifolia leaf extract has lately been employed for biosynthesizing AuNPs due to its tremendous potential [39]. 20 g of pulverized dried leaves of V. betonicifolia was mixed with 150 ml of DI water and stirred at 60°C for 60 min to obtain leaves extract of V. betonicifolia and then filtered. The filtrate was collected and stored characterization, antimicrobial, and antioxidant activities at 4°C [43]. The biosynthesis of Au NPs, 1 mM of HAuCl43H2O was added to 25 ml of leaves extract of V. betonicifolia, the resulting mixture was heated at 40°C for 60 min with stirring. When the color change from yellow to ruby red, the formation of AuNPs was revealed. The AuNPs biosynthesized were separated by centrifugation from the reaction mixture, and were washed, dried and further calcined at 200°C for 3 h [39]. The synthesis mechanisms during the biosynthesis process suggested that, the polyphenols and flavonoids might be functioned as reducing the gold ions to zero- valent species via reduction and oxidation reaction with the production of keto form products. Furthermore, other secondary metabolites (surfactants, proteins, alkaloids, etc.) present in the leaf extract of the V. betonicifolia simultaneously stabilized and capped the zero-valent species of Au0, leading to the formation of nanoscale AuNPs (Figure 13) [39,58]. Au NPs were characterized by XRD, EDX, TEM, DLS, FTIR. Additionally, the Antimicrobial properties of the AuNPs were further explored against bacterial and mycological species, antioxidant, cytotoxic, and cytobiocompatibility properties were examined in vitro against linoleic acid peroxidation, MCF-Cancer cells, and human mesenchymal stem cells (hMSCs), respectively (Table 1). The antibacterial performance of VB-Au NPs was determined in terms of log reduction and % killing efficiency of bacterial strains. The results have been shown that the synthesized Au NPs displayed 4.14, 4.32, 4.50, and 4.65 log10 reductions in CFU of S. aureus, B. subtilis, E. coli, and P. aeruginosa, respectively, with more than 82% killing efficiency. AuNPs have shown a more significant inhibitory effect on Gram- negative than on Gram-positive bacterial species. Base on live/dead staining and the intracellular ROS generation confirmed that the destruction of the microbial cell membrane and subsequent cell death induced by AuNPs were caused by the membrane permeabilization and production of ROS species. The results of antifungal activity revealed that Au NPs significantly reduced the CFU of C. albicans, A. fumigatus, A. flavus, and A. niger by 3.32, 4.0, and 3.55 log10 reductions, respectively. The cytotoxicity efficacy of AuNPs against MCF-7 cells at the therapeutic potential concentration of 120 μg/mL was dose-dependent. The results indicated that Au NPs seem to be the most lethal to MCF-7 cancer cells, destroying nearly all cancerous cells. Antioxidant activity of Au NPs compared to the leaves extract of Viola betonicifolia, and external standard (BHT). The anti- linoleic acid peroxidation activity ofAuNPs was superior to that of Viola betonicifolia leaves extract, although a little less than BHT. The biocompatibility of VB-Au NPs was assessed with the hMSCs in vitro compared to the V. betonicifolia leaves extracts, Au NPs cell viability (82.60%) with hMSCs and cell viability of V. betonicifolia leaves (86.5%), this because V. betonicifolia leaves contains biocompatible secondary metabolites that demonstrated excellent biocompatibility.
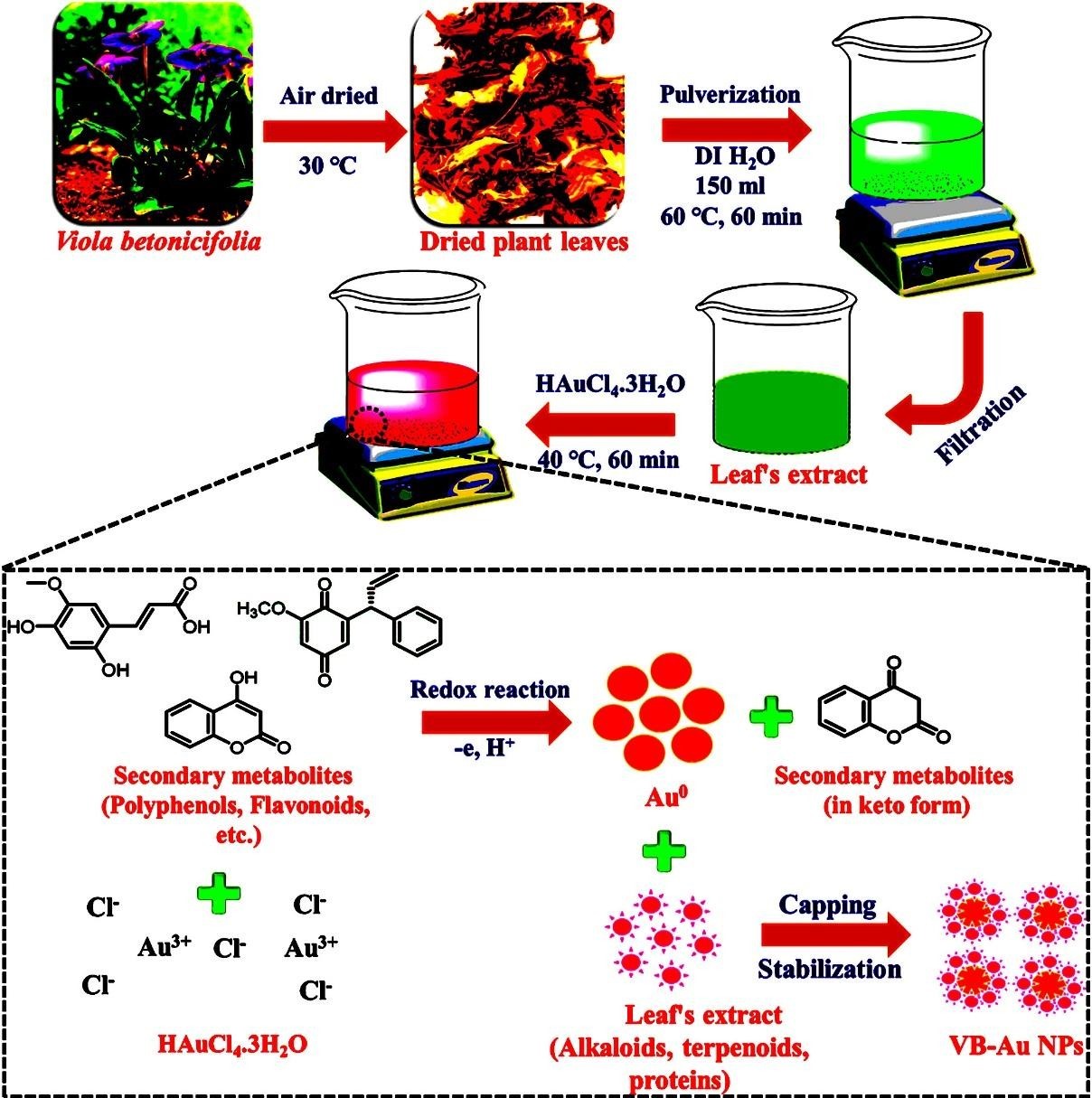
Figure 13: Schematic demonstration and possible synthesis mechanism for the biosynthesis of AuNPs with leaves of V. betonicifolia [58].
Couroupita guianensis Aubl - PdNPs
Couroupita. guianensis Aubl. (C. guianensis), commonly known as ayahuma and cannonball tree, belongs to Lecythidaceae family and possesses many medicinal properties such as antibiotic, antifungal, anticancer, antiulcer, antiseptic, and analgesic qualities. Extracts from this tree were used to cure colds and stomach aches and juice made from the leaves were used to cure malaria [13]. Generally, nanomaterials among them PdNPs have gained colossal attention due to their indispensable unique physio-chemical properties relative to their macro scale counterparts [2]. Also, these nanomaterials have shown extraordinary pharmaceutical applications like drug delivery, imaging, cell labelling, Surface Enhanced Raman Scattering (SERS) detection, antioxidant, antifl ammatory, bactericidal and cancer theranostics [9]. Recently, biosynthesized PdNPs using the aqueous fruit extract of C. guianensis Aubl as a potent biological reducing agent have been confirmed. In fact, 10 g of sterilized C. guianensis fruit powder was mixed with 200 of Milli Q water and kept in boiling water bath at 60oC for 20 min and filtered. To biosynthesize PdNPs 5 mL of the extract was blended with 95 mL of 1 Mmol substrate (PdCl2) and continuous stirring, the reaction mixture was checked for intense blackish colour formation, the mixture was centrifugated, filtered and resuspended in Mili-Q water and then used for characterization by UV vis, FTIR, XRD, TEM, HRTEM. The findings revealed monodispersed spherical PdNPs with an average diameter of 6 nm, ranging in size from 5 to 15 nm. The possible biomolecules as phenolic and reducing sugars present in the fruit extract is responsible for reduction and stabilization of PdNPs. The antibacterial effect of synthesized PdNPs were studied against bacterial human pathogens PdNPs exhibits high level of bactericidal effect against K. pneumonia, E. coli, S. typhi and V. cholera [22]. The PdNPs biosynthesized have shown remarkable antibacterial activity against both gram positive and gram-negative bacterial pathogens at 25 mg/ml dosage. The exact mechanism of Nano metals triggered antibacterial action are still being investigated, there are two basic phenomena has been proposed, fi rstly the toxicity arising due to the dissolution of metals from surface of nanoparticles and also oxidative stress via the generation of Reactive Oxygen Species (ROS) on surfaces of the nanoparticles. Moreover, in vitro cell viability (MTT) assay on A549 lung cancer cells was carried out to assess anticancer effi cacy of synthesized PdNPs in different doses and to assess the biocompatibility of PdNPs hemolytic assay was performed upon calculating the damage to human RBCs. The PdNPs exhibited extraordinary anticancer properties and the blood compatibility in dose-dependent manner. The IC50 values of PdNPs were determined to be 121 mg/ml for 24 h treatment period. The results showed that at 100 mg/ml concentration PdNPs (2.7%) exhibits comparatively very less acceptable level of red hemoglobin release than positive control Triton-1X (100%), implying its safe nature in application (Table 1). The synthesized PdNPs can be used as multifunctional hybrid nanomaterial for a wide spectrum of biomedical application.
Conclusions
The biosynthesis of NPs has recently advanced, resulting in a new strategy in biomedical applications. For the biosynthesis of nanoparticles such as Au, Ag, Au and Ag, Pd, FeO, NiO, and MnO2-NPs, plant extracts containing biologically active phytomolecules act as both reducing and capping/stabilizing agents for a variety of biosynthesized such nanoparticles. Because of the diversity in metabolite composition, the size, shape, and morphology of the nanoparticles varied, which influenced their overall properties and activities. Nanoparticles derived from plant extracts have the potential to be widely employed in nanobiotechnology, including as antibacterial and antifungal agents in bandages, insecticides for agricultural crops, and drugs for the treatment of rare diseases, including some types of cancer. Further studies are required to confirm the mechanisms and pathways involved in the biosynthesis of these NPs as well as their potential use in biomedicine.
Author Statements
Conflict of Interest
The author declare no conflict of interest
Funding
There is no institutional funding for this project.
References
- Abbasi BA, Iqbal J, Mahmood T, Qyyum A, Kanwal S. Biofabrication of iron oxide nanoparticles by leaf extract of Rhamnus virgate: characterization and evaluation of cytotoxic, antimicrobial and antioxidant potentials. Appl Organometal Chem. 2019; 33: e4947, 1-15.
- Al-Dhabi NA, Balachandran C, Raj MK, Duraipandiyan V, Muthukumar C, Ignacimuthu S, et al. Antimicrobial: antimycobacterial and antibiofi lm properties of Couroupita guianensis Aubl. fruit extract. BMC Complement Altern Med. 2012; 12: 242.
- Bindhu MR, Umadevi M. Antibacterial activities of green synthesized gold nanoparticles. Mater Lett. 2014; 120: 122-5.
- Boomi P, Ganesan RM, Poorani G, Gurumallesh Prabu HG, Ravikumar S, Jeyakanthan J. Biological synergy of greener gold nanoparticles by using Coleus aromaticus leaf extract. Mater Sci Eng C Mater Biol Appl. 2019; 99: 202-10.
- Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog. 2006; 22: 577-83.
- Chung IM, Park I. Hyun K.S. Thiruvengadam M, Rajakumar G, plant- mediated synthesis of silver nanoparticles: their characteristic properties and therapeutic applications. Nanoscale Res Lett. 2016; 11: 40.
- Chung IM, Abdul Rahuman A, Marimuthu S, Kirthi AV, Anbarasan K, Padmini P et al. Green synthesis of copper nanoparticles using Eclipta prostrata leaves extract and their antioxidant and cytotoxic activities. Exp Ther Med. 2017; 14: 18-24.
- Contado C. Nanomaterials in consumer products: A challenging analytical problem. Front Chem. 2015; 3: 48.
- Dumas A, Couvreur P. Palladium: a future key player in the nanomedical fi eld Chem. sci. Chem Sci. 2015; 6: 2153-7.
- Elbagory AM, Hussein AA, Meyer M. The in vitro immunomodulatory effects of gold nanoparticles synthesized from Hypoxis hemerocallidea aqueous extract and hypoxoside on macrophage and natural killer cells. Int J Nanomedicine. 2019; 14: 9007-18.
- Fayaz AM, Balaji K, Girilal M, Yadav R, Kalaichelvan PT, Venketesan R, et al. Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov Today. 2010; 17: 1044-52.
- Gharehyakheh S, Ahmeda A, Haddadi A, Jamshidi M, Nowrozi M, Zangeneh MM, et al. Effect of gold nanoparticle synthesized using the aqueous extract of Satureja hortensis leaf on enhancing the shelf life and removing Escherichia coli O157: h7 and Listeria monocytogenes in minced camel’s meat: the role of nanotechnology in the food industry. Appl Organomet Chem. 2020; 34: e5492.
- Gnanasekar S, Murugaraj J, Dhivyabharathi B, Krishnamoorthy V, Jha PK, Seetharaman P, et al. Antibacterial and cytotoxicity effects of biogenic palladium nanoparticles synthesized using fruit extract of Couroupita guianensis Aubl. J Appl Biomed. 2018; 16: 59-65.
- Kumar V, Yadav SK. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol. 2009; 84: 151-7.
- Gopinath V, Priyadarshini S, Al-Maleki AR, Alagiri M, Yahya R, Saravanan S et al. In vitro toxicity, apoptosis and antimicrobial effects of phyto-mediated copper oxide nanoparticles. RSC Adv. 2016; 6: 110986-95.
- Hirpara DG, Gajera HP. Green synthesis and antifungal mechanism of silver nanoparticles derived from chitin- induced exometabolites of Trichoderma interfusant. Appl Organomet Chem. 2020; 34: e5407.
- Huang L, Weng X, Chen Z, Megharaj M, Naidu R. Synthesis of iron-based nanoparticles using oolong tea extract for the degradation of malachite green. Spectrochim Acta A Mol Biomol Spectrosc. 2014; 117: 801-4.
- Ibrahim S, Charinpanitkul T, Kobatake E, Sriyudthsak M. Nanowires nickel oxide and nanospherical manganese oxide synthe sized via low temperature hydrothermal technique for hydrogen peroxide sensor. J Chem. 2016; 2016 :1-6.
- Ijaz F, Shahid S, Khan SA, Ahmad W, Zaman S. Green synthesis of copper oxide nanoparticles using Abutilon indicum leaf extract: antimicrobial, antioxidant and photocatalytic dye degradation activities. Trop J Pharm Res. 2017; 16: 743-53.
- Iqbal S, Bhanger MI, Anwar F. Antioxidant properties and components of some commercially available varieties of rice bran in Pakistan. Food Chem. 2005; 93: 265-72.
- Kalaiarasi A, Sankar R, Anusha C, Saravanan K, Aarthy K, Karthic S et al. Copper oxide nanoparticles induce anticancer activity in A549 lung cancer cells by inhibition of histone deacetylase. Biotechnol Lett. 2018; 40: 249-56.
- Khan SA, Lee C-S-S. Green biological synthesis of nanoparticles and their biomedical applications. In: Springer Science and Business Media B.V. 2020a; 10: 247-80.
- Khan SA, Shahid S, Lee CS. Green Synthesis of Gold and Silver Nanoparticles Using Leaf Extract of Clerodendrum inerme, characterization, antimicrobial, and antioxidant activities. Biomolecules. 2020b; 10: 835-42.
- Khan M, Khan M, Kuniyil M, Adil SF, Al-Warthan A, Alkhathlan HZ, et al. Biogenic synthesis of palladium nanoparticles using Pulicaria glutinosa extract and their catalytic activity towards the Suzuki coupling reaction. Dalton Trans. 2014; 43: 9026-31.
- Khan SA, Shahid S, Ayaz A, Alkahtani J, Elshikh MS, Riaz T. Phytomolecues-Coated NiO nanoparticles synthesis using Abutilon indicum leaf extract: antioxidante, antibacterial, and anticancer activities. Int J Nanomedicine. 2021; 2021: 1757-73.
- Khan SA, Noreen F, Kanwal S, Iqbal A, Hussain G. Green synthesis of ZnO and Cu-doped ZnO nanoparticles from leaf extracts of Abutilon indicum, Clerodendrum infortunatum, Clerodendrum inerme and investigation of their biological and photocatalytic activities. Mater Sci Eng C Mater Biol Appl. 2018; 82: 46-59.
- Khan SA, Shahid S, Shahid B, Fatima U, Abbasi SA. Green synthesis of MnO nanoparticles using Abutilon indicum Leaf extract for biological, photocatalytic, and adsorption activities. Biomolecules. 2020; 10: 785.
- Kolahalam LA, Prasad KRS, Murali Krishna P, Supraja N. Saussurea lappa plant rhizome extract-based zinc oxide nanoparticles:synthesis, characterization and its antibacterial, antifungal activities and cytotoxic studies against Chinese hamster ovary (CHO) cell lines. Heliyon. 2021; 7: e07265.
- Korbekandi H, Iravani S, Abbasi S. Production of nanoparticles using organisms. Crit Rev Biotechnol. 2009; 29: 279-306.
- Kumar D, Kumar K, Gupta J, Bishnoi N, Kumar S. A mini review on chemistry and biology of Holoptelea integrifolia Roxb. Planch (Ulmaceae). Asian Pac J Trop Biomed. 2012; 1691: S1200-5.
- Kumar V, Singh S, Singh A, Dixit AK, Srivastava B, Sidhu GK et al. Phytochemical, antioxidant, antimicrobial, and protein binding qualities of hydro-ethanolic extract of Tinospora cordifolia. J Biol Act Prod Nat. 2018; 8: 192-200.
- Kumar V, Singh S, Srivastava B, Bhadouria R, Singh R. Green synthesis of silver nanoparticles using leaf extract of Holoptelea integrifolia and preliminary investigation of its antioxidant, anti-infl ammatory, antidiabetic and antibacterial activities. J Environ Chem Eng. 2019; 7.
- Kumar V, Yadav SK. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol. 2009; 84: 151-7.
- Kuo PC, Yang ML, Wu PL, Shih HN, Thang TD, Dung NX, et al. Chemical constituents from Abutilon indicum. J Asian Nat Prod Res. 2008; 10: 699-703.
- Letchumanan D, Sok SPM, Ibrahim S, Nagoor NH, Arshad NM. Plant- based biosynthesis of copper/copper oxide nanoparticles: an update on their applications in biomedicine, mechanisms, and toxicity. Biomolecules. 2021; 11: 564.
- Lian S, Diko CS, Yan Y, Li Z, Zhang H, Ma Q et al. Characterization of biogenic selenium nanoparticles derived from cell-free extracts of a novel yeast Magnusiomyces ingens. 3 Biotech. 2019; 9: 221.
- Lu H, Zhang X, Khan SA, Li W, Wan L. Biogenic synthesis of MnO2 nanoparticles with leaves extract of viola betonicifolia for enhanced antioxidante, antimicrobial, cytoxic, and biocompatible applications. Front Microbiol. 2021; 12: 761084.
- Majdalawieh A, Kanan MC, El-Kadri O, Kanan SM. Recent advances in gold and silver nanoparticles: synthesis and applications. J Nanosci Nanotechnol. 2014; 14: 4757-80.
- David L, Moldovan B, Vulcu A, Olenic L, Perde-Schrepler M, Fischer-Fodor E et al. Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids Surf B Biointerfaces. 2014; 122: 767-77.
- Muhammad N, Saeed M, Khan H. Antipyretic, analgesic and anti-inflammatory activity of Viola betonicifolia whole plant. BMC Complement Altern Med. 2012; 12: 59.
- Muthukumar T, Sudhakumari B, Sambandam B, Aravinthan A, Sastry TP, Kim J. Green synthesis of gold nanoparticles and their enhanced synergistic antitumor activity using HepG2 and MCF7 cells and its antibacterial effects. Process Biochem. 2016; 51: 384-91.
- Parhi P, Mohanty C, Sahoo SK. Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov Today. 2012; 17: 1044-52.
- Parthiban E, Manivannan N, Ramanibai R, Mathivanan N. Green synthesis of silver-nanoparticles from Annona reticulata leaves aqueous extract and its mosquito larvicidal and anti-microbial activity on human pathogens. Biotechnol Rep (Amst). 2019; 21: e00297.
- Raj Preeth DR, Shairam M, Suganya N, Hootan R, Kartik R, Pierre K et al. Green synthesis of copper oxide nanoparticles using sinapic acid: an underpinning step towards antiangiogenic therapy for breast cancer. J Biol Inorg Chem. 2019; 24: 633-45.
- Ramirez-Perez JC. Aerobic biodegradation kinetics of solid organic wastes. 1st ed. Lambert Academic Publishing, ISBN 978-3-659-46135-4; 2013.
- Ramirez-Perez JC, Alves dosReis T, Rizzutto M. Identifying and detecting entomopathogenic fungi using Surface-enhanced Raman spectroscopy, Brazilian Journal of Animal and Environmental Research. 2021; 4: 4832-50.
- Ramirez-Perez JC, Olivera AT, C, Rizzutto M. Impact of silver nanoparticles size on SERS detection and identification of filamentous fungi, Spectroschimica acta Part A: molecular and Biomolecular. Spectroscopy. 2022; 272: 120980.
- Rehana D, Mahendiran D, Kumar RS, Rahiman AK. Evaluation of antioxidant and anticancer activity of copper oxide nanoparticles synthesized using medicinally important plant extracts. Biomed Pharmacother. 2017; 89: 1067-77.
- Rehman S, Ansari MA, Alzohairy MA, Alomary, Jermy, Shahzad, et al. Antibacterial and antifungal activity of novel synthesized neodymium-substituted cobalt ferrite nanoparticles for biomedical application. Processes. 2019; 7: 714.
- Rizwan K, Khan SA, Ahmad I, Rasool N, Ibrahim M, Zubair M, et al. A comprehensive review on chemical and pharmacological potential of Viola betonicifolia: A plant with multiple benefits. Molecules. 2019; 24: 3138.
- Di Pilato V, Arena F, Tascini C, Cannatelli A, Henrici De Angelis L, Fortunato S et al. mcr-1.2, a new mcr variant carried on a transferable plasmid from a colistin-resistant KPC carbapenemase-producing Klebsiella pneumoniae strain of sequence type 512. Antimicrob Agents Chemother. 2016; 60: 5612-5.
- Saranya D, Sekar J. GC-MS and FT-IR analyses of ethyl acetate leaf extract of Abutilon indicum (L.) sweet. Int J Adv Res Biol Sci. 2016; 3: 193-7.
- Shah M, Fawcett D, Sharma S, Tripathy SK, Poinern GEJ. Green synthesis of metallic nanoparticles via biological entities. Materials (Basel). 2015; 8: 7278-308.
- Sulaiman GM, Tawfeeq AT, Jaaffer MD. Biogenic synthesis of copper oxide nanoparticles using Olea europaea leaf extract and evaluation of their toxicity activities: an in vivo and in vitro study. Biotechnol Prog. 2018; 34: 218-30.
- Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomedicine. 2010; 6: 257-62.
- Velsankar K, Kumar AK, Preethi R, Muthulakshmi V, Sudhahar S. Green synthesis of CuO nanoparticles via Allium sativum extract and its characterizations on antimicrobial, antioxidant, antilarvicidal activities. J Environ Chem Eng. 2020; 8.
- Mendhulkar VD, Yadav A. Anticancer activity of Camellia sinensis mediated copper nanoparticles against HT-29, MCF-7, and MOLT-4 human cancer cell lines. Asian J Pharm Clin Res. 2017; 10: 82-8.
- Wang M, Meng Y, Zhu H, Hu Y, Xu C, Li W et al. Green synthesized gold nanoparticles using viola betonicifolia leaves extract:characterization, antimicrobial, antioxidante, and cytobiocompatible activities. Int J Nanomedicine. 2021; 16: 7319-7337.
- Yasin SMM, Ibrahim S, Johan MR. Effect of zirconium oxide nanofi ller and dibutyl phthalate plasticizer on ionic conductivity and optical properties of solid polymer electrolyte. ScientificWorldJournal. 2014; 2014: 547076.
- Zakaria N, Mahdzir MA, Yusoff M, Mohd Arshad N, Awang K, Nagoor NH. Cytotoxic effects of Pinnatane a extracted from Walsura pinnata (Meliaceae) on human liver cancer cells. Molecules. 2018; 23: 2733.
- Zhang Z, Chen Z, Cheng F, Zhang Y, Chen L. Highly sensitive on-site detection of glucose in human urine with naked eye based on enzymatic- like reaction mediated etching of gold nanorods. Biosens Bioelectron. 2017; 89: 932-6.
- Zhang Z, Wang J, Nie X, Wen T, Ji Y, Wu X, et al. Near infrared laser-induced targeted cancer therapy using thermoresponsive polymer encapsulated gold nanorods. J Am Chem Soc. 2014; 136: 7317-26.
- Zhao HW, Su HT, Ahmeda A, Sun YQ, Li ZY, Zangeneh MM et al. Biosynthesis of copper nanoparticles using Allium Eriophyllum Boiss leaf aqueous extract; characterization and analysis of their antimicrobial and cutaneous wound- healing potentials. Appl Organomet Chem. 2022; 36.
- Zhao P, Li N, Astruc D. State of the art in gold nanoparticle synthesis. Coord Chem Rev. 2013; 257: 638-65.
- Zhou Y, Wang CY, Zhu YR, Chen ZY. A novel ultraviolet irradiation technique for shape- controlled synthesis of gold nanoparticles at room temperature. Chem Mater. 1999; 11: 2310-2.