
Research Article
Ann Hematol Oncol. 2015;2(4): 1034.
Management of Postransplant Relapse and Persistent Disease in Lymphoid Malignancies: Exploring the Graft versus Lymphoma Effect
Cabrero M, López-Godino O, López-Corral L, Martín A, García-Sanz R, Mateos MV, Perez Lopez E, Labrador J, Vázquez L, Sanchez-Guijo F, Del Caņizo C and Caballero D*
Department of Hematology, Hospital Universitario Salamanca, Spain
*Corresponding author: Dolores Caballero, Department of Hematology, Hospital Universitario Salamanca-IBSAL, Spain
Received: January 26, 2015; Accepted: April 21, 2015; Published: April 27, 2015
Abstract
Allogenic hematopoietic Stem Cell Transplantation (AlloSCT) is potentially a curative option for relapse or refractory lympho proliferative disorders, based in part on the Graft Versus Lymphoma Effect (GVLE). However, the role of GVLE enhancement and the means of implementing it effectively in relapse or persistent disease after AlloSCT remain unclear. We report the evaluation of 26 patients with post-AlloSCT relapse or persistent disease in a series of 112 AlloSCTs. In 19/26 (73%) patients, GVLE was enhanced by tapering immunosuppressive treatment (IST) and/or Donor Lymphocyte Infusion (DLI), achieving a response in 13 (68%), 11 of which were Complete Remissions (CRs). With respect to histology’s, an immune-mediated response was observed in 6/6 patients with NHL, 5/7 with CLL and 2/6 with HL. Graft Versus Host Disease (GVHD) appeared in 16/19 (84%); only one death was ascribed to GVHD. The remaining 7/26patients, in whom GVLE enhancement was not possible due to GVHD, received conventional chemotherapy with or without radiotherapy; two patients achieved CR and one achieved Partial Remission (PR).
Four-Year Progression-Free Survival (PFS) and Overall Survival (OS) after treatment of relapse/refractory patients were 40% and 45%,respectively (median follow-up, 56 months; range 11-138 months).
Disease response to immune manipulation proves the existence of GVLE, which appears in all histological subtypes, although less frequently in HL. GVLE enhancement enables us to ensure maintained responses, with PFS and OS similar to those in non-relapsing patients. Therefore, the immune approach should always be considered as a treatment for relapse/refractory patients.
Keywords: Chronic lymphocytic leukemia; Non-hodgkin lymphoma; Hodgkin lymphoma
Introduction
Allogenic hematopoietic stem cell transplantation (AlloSCT) is potentially a curative option for refractory or relapsed lympho proliferative disorders such as Non-Hodgkin Lymphoma (NHL), Hodgkin Lymphoma (HL) and Chronic Lymphocytic Leukemia (CLL). The Reduced-Intensity Conditioning (RIC) regimen has lowered the high non-relapse mortality rate previously reported with conventional AlloSCT, so this procedure can be offered to patients who are older, with co morbidities or who have received a previous treatment that excludes them from a myeloablative conditioning regimen. The curative effect of AlloSCT is mainly based on the potential occurrence of the immune Graft Versus Lymphoma Effect (GVLE).It can overcome adverse prognostic factors, allowing patients to achieve long-term Disease-Free Survival (DFS) [1] .The existence of GVLE is supported by the lower relapse rate associated with the development of chronic Graft Versus Host Disease (GVHD) as well as the long-term remissions observed after Donor Lymphocyte Infusions (DLIs) in patients with lymphoid malignancies who relapse after AlloSCT [2,3].
The relapse rate after AlloSCT is highly variable in lymph proliferative disorders, ranging between 20 and 70%, depending on histological subtype and clinical status [4-7]; it is clear that, if the GVLE is the main goal of allogenic transplant, it should be enhanced if relapse occurs. However, few reports have focused on GVLE enhancement for treating relapse after AlloSCT [8]. Accordingly, there is no consensus about the best strategy for managing post- AlloSCT relapsed or persistent disease, and the most effective means of fuelling up GVLE remains unclear.
In the present study, we evaluate how GVLE enhancement, through tapering Immune Suppressive Treatment (IST) and/or DLI, is able to control the disease in patients with lymphoid malignancies who relapse after AlloSCT or whose disease persists by day +100.
Patients and Methods
Starting with our AlloSCT database of 112 patients who received an AlloSCT between January 1999 and December 2012, we selected those with NHL, HL or CLL. The current indications for AlloSCT in our center are: 1) patients with aggressive NHL who do not achieve at least a Partial Response (PR) after two lines of chemotherapy or relapse after Autologous Stem Cell Transplant (ASCT); 2) patients within do lent lymphomas and several relapses; 3) patients with HL who relapse after ASCT; 4) poor-risk CLL according to the 2007 EBMT consensus (17p deletion and/or resistance to purine analogues) [9].
From all patients with CLL, NHL or HL who consecutively underwent Reduced Intensity Conditioning (RIC) followed by AlloSCT, we identified those who had not achieved Complete Remission (CR) by day +100 or who had relapsed after AlloSCT, and thereby managed to recruit 26 patients.
We collected data from all 26 patients about the treatment applied and their response. Baseline clinical data were previously reported in the EBMT-group database and additional information from patient medical records was collected. Every patient and donor signed a consent form before AlloSCT.
Our primary objective was to evaluate whether potentiating of GVLE can contribute to control the disease in patients with persistent disease at day +100 or who had relapsed after AlloSCT. A secondary objective was to evaluate progression-free survival and Overall Survival (OS) in the whole series of patients, recalculating the outcome in a second step after GVLE enhancement in those patients who relapsed or progressed after AlloSCT.
Response and Graft Versus Host Disease Assessment
Disease response was assessed by chest-abdomen-pelvis Computerized Tomography (CT) or Positron Emission Tomography (PET), and bone marrow biopsy if necessary, according to standard criteria [10] on day +100, or earlier if progression or relapse was suspected. Thereafter, the disease was evaluated on the basis of clinical criteria according to the standard of care at our center, with radiological evaluations every 3 months during the first year post-AlloSCT. In relapsed/persistent disease, at least one complete evaluation was undertaken after each treatment line had been completed.
GVHD diagnosis was based on clinical and histological criteria. Acute GVHD was classified as grade 0 to IV according to the international standards [11], and chronic GVHD was defined as mild, moderate or severe according to the recommendations of the NIH working group, which includes the category of acute lateonset GVHD (features of acute GVHD appearing after day +100) and overlap syndrome (diagnostic or distinctive features of chronic GVHD and acute GVHD appearing together) [12].
Statistical Analysis
Statistical analyses were done using SPSS version 20.0 (SPSS, Chicago, IL, USA).
Frequencies among the classes of categorical variables were examined with the chi-square test. OS and PFS were estimated by the Kaplan–Meier (KM) method, and KM curves were compared by the log-rank test.
OS was defined as the time from the date of AlloSCT to death from any cause. Progression-free survival 1 (PFS-1) was taken as the time from the date of AlloSCT to death, progression or relapse, considering the first relapse or progression of the disease after AlloSCT as an event. PFS-2 was calculated as the time from AlloSCT to death, relapse or disease progression, but not considering the first relapse or progression as an event if it was controlled by GVLE enhancement.
Results
Between 1999 and 2012, 112 patients with NHL, HL or CLL received a RIC AlloSCT in our Transplant Unit(13 diffuse large B cell lymphomas(11.6%), 22 indolent NHLs(19.6%), 25 CLLs (22.3%), 18 T cell lymphomas (TCLs) (16.1%), 10 mantle cell lymphomas(8.9%) and 24 HLs(21.4%)). The donor was unrelated in 34 patients (30.4% of cases). With a median follow-up of 58 (5-127) months, OS and PFS were 55.4% and 48.2%, respectively. The overall Non-Relapse Mortality (NRM) rate was 21.4%.
From this whole cohort, we recruited 26 patients with persistent disease at day +100 (n=11; 42.3%) or relapsed after AlloSCT (n=15; 57.7%) for the present study. Their baseline characteristics are summarized in Table 1.
Global (n=26)
Age, years [median (range)]
47.5 (17-65)
Male patients [n (%)]
21 (80.8)
≥ 3 previous treatment lines [n (%)]
18 (69.2)
Previous ASCT (excluding CLL patients, n=18) [n (%)]
10 (55.6%)
Months from diagnosis to HSCT [median (range)]
24 (5-115)
Related donor
HLA-matched donor
20 (76.9%)
21 (80.8%)
Diagnosis
Diffuse large B cell NHL
Mantle cell lymphoma
Hodgkin lymphoma
CLL
T-NHL
Indolent lymphoma
2 (7.7%)
2 (7.7%)
9 (34.6%)
8 (30.8%)
2 (7.7%)
3 (11.5%)
Status at HSCT [n (%)]
CR
16 (34)
PR
23 (48.7)
Less than PR
8 (17)
Months from AlloSCT to relapse or +100 day reevaluation with persistent disease [median (range)]
4 (1-75)
Acute GVHD (all grade 1-2)
20 (77%)
Table 1: Main patient characteristics.
Transplant Characteristics
All patients received a RIC regimen consisting of intravenous Fludarabine 30 mg/m2 from days -7 to -3, and Melphalan 70 mg/ m2 on days -3 and -2. One patient was participating in a phase II clinical trial [13] (Clinical Trials Identifier (CTI): NCT00644371) and received radio immunotherapy with Zevalin (0.4 mCi/kg) on day -14 as part of the conditioning regimen.
The donor was unrelated in six patients (23%); 21 patients (81%) received an AlloSCT from an HLA-matched related or unrelated donor.
GVHD prophylaxis consisted of cyclosporine (0.5 mg/kg in continuous infusion from day -7 to -2, and 1 mg/kg from day -1) and methotrexate (15 mg/m2 on day +1 and 10 mg/m2 on days +3, +6 and +11) in all except four patients, who received tacrolimus and sirolimus in the context of a multicenter clinical trial 14 (CTI: NCT00641632).
Donor Lymphocyte Infusion
DLI were given in an escalating protocol with a rest period of at least 28 days between each, in the absence of GVHD or disease response to the previous DLI. The initial doses given by sibling donors and unrelated donors were 1 x 107 and 1 x 106 CD3-positive cells/kg, respectively. Second DLI doses were 3.2 x 107 and 3.2 x 106 CD3-positive cells/kg for related and unrelated donors, respectively. The third DLI dose was 1 x 108 and 1 x 107 CD3-positive cells/kg in sibling and unrelated donors, respectively.
Treatment and Response
Treatment strategies and responses are schematized in Figure 1.

Figure 1: Treatment schema.
Immune manipulation
Consisting of tapering immune suppression and/or DLI, is the standard of care in our center in the case of relapse or persistence of the disease, except in the case of recent or active severe GVHD. This strategy was adopted to control the disease in 19 of these 26 patients (73.1%), achieving a response in 68.4% of them.
Tapering immune suppression was done in 13 patients at the moment of relapse (n=11) or when the disease persisted at day +100 (n=2). IST was tapered for a median of 22 days (range, 1-219 days) from initial reduction to complete withdrawal. Afterwards, four patients received donor lymphocyte infusions (DLI) because of the lack of GVHD/GVLE after ending the IST. All but two of them developed GVHD with a median of 98 days (range, 20-392 days), eight patients after withdrawal of IST alone and three after withdrawal of IST followed by DLI; the disease was controlled in 9/13 patients (69.2%): seven with CR and two with PR.
DLI as initial treatment was planned for six patients who had already finished IST at relapse. All but one of them developed GVHD with a median of 60 days (range, 7-110 days), and four of them achieved CR. In total, 10 patients received DLI; one, two and three DLI doses in four, one and five patients, respectively.
In addition, 3/19 patients(15.8%) had received conventional chemotherapy to reduce tumor burden before the immune approach was applied.
Other treatments
GVLE induction was not considered appropriate for seven patients due to the presence of active GVHD (5 cases) or prior severe GVHD (2 cases). Five patients received conventional chemotherapy and two receivedanti-CD20 monoclonal antibody. Three patients required local radiotherapy as an additional treatment.
In summary, after adopting the immune approach or chemotherapy, 16 of the 26 patients who relapsed or whose disease persisted at day +100 responded (ORR 61.5%) and 13 achieved CR (50.0%).
If we consider only the group of 19 patients in whom GVLE was induced, 13 responded (68.4%), 11 achieving CR (57.9%) and two are achieving PR (10.5%).
The median time from immune manipulation to response was 3.5 months (range, 2-36 months). Regarding specific strategies, 7/9 patients responded (77.8%) after tapering IST and 5 achieved CR (55.6%). After the combination of withdrawal of IST and DLI, 2/4 responded (50.0%) and all achieved CR. After DLI alone, 4/6 (66.7%) responded, all of them with CR. Concerning responses after GVLE enhancement and histological subtype, all six patients with NHL achieved CR after immune manipulation. Among the seven CLLs, ORR was 71.4% (n=5), with 57.1% CR (n=4); in the six HL patients, 33.3% of patients responded (n=2) and 1 CR and 1 PR were observed (16.7% each).
In the group of seven patients treated with chemotherapy, the ORR was 42.9% (n=3), with 2 CR (28.6%) and 1 PR (14.3%).
Graft versus Lymphoma Effect and Graft Versus Host Disease
After immune manipulation, 16/19 patients (84.2%) developed GVHD (beginning as acute GVHD symptoms in three cases, overlap syndrome in six and chronic GVHD symptoms in seven), and it was associated with GVLE in 13/19 patients (68.4%). All of these 13 patients with effective GVLE presented cGVHD symptoms, 46.2% of which were mild/moderate and 53.8% were severe cGVHD.
The response quality was not correlated with the severity of chronic GVHD. Thus, the CR rate was 71% in patients with severe cGVHD and 100% in patients with mild/moderate cGVHD (p=0.115). However, patients who developed severe cGVHD had a lower risk of relapse/progression compared with mild/moderate cGVHD (14% vs. 67%, respectively; p=0.05).
At the final follow-up, of the 11 patients who were alive, four remained on IST. Chronic GVHD was in CR in 21.1% (4/19), while the remaining 15 patients (78.9%) had active but tolerable cGVHD at the final follow-up (the poorest ECOG performance status was just 1).
To analyze the time between cGVHD onset and disease status, we examined cumulative cGVHD incidence and prevalence in patients with active disease, observing that an increase in cGVHD was correlated with a decrease in the percentage of patients with active disease (Figure 2).
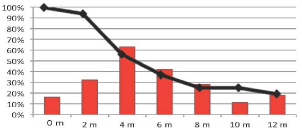
Figure 2: Relationship between cumulative cGVHD incidence (red bars) and
disease response over time (months). Black line represents percentage of
patients with active disease, that decrease in inverse proportion to the chronic
GVHD development.
Survival
After a median follow-up of 56months (range, 11-138 months), the estimated 4-year OS was45% for the whole series of 26 patients. The PFS-1 of these 26 patients, considered for the study because of relapsing or persistent disease was 10% at 4 years. After GVLE enhancement, 13 patients responded, and in a second step, the PFS-2 was calculated, discounting events of those patients in whom a response was achieved. The PFS-2 was 40% at 4 years, reaching a plateau at 36 months post-AlloSCT (Figure 3).

Figure 3: Improvement in PFS with GVLE. Dashed and continuous lines
represent PFS1 and PFS2 after GVLE, respectively.
At the final follow-up, 11 of the 26 patients (42.3%) were alive, 9 (34.6%) of whom disease-free; 47.4% (9/19) of patients in whom immune manipulation was performed were alive, compared with only 28.6% (2/7) of those who received conventional treatment because it was not possible to adopt an immune approach. Fifteen patients (57.7%) died, disease progression being the primary cause, irrespective of the treatment received (12/15).
The univariate analyses indicated that the development of cGVHD after immune manipulation (4-year PFS232% vs. 0%; P=0.017), severe cGVHD (4-year PFS2 51% vs. 25%; P=0.05) and different diagnosis of HL (4-year PFS235% vs.17%; P=0.016) had a significant influence on PFS. Early or late relapse, previous development of acute GVHD, status of disease at transplant and inclusion criteria (relapse vs. persistence of disease) showed no significant association with PFS.
Discussion
In the present study we show how GVLE enhancement through tapering IST and/or DLI is able to control the disease in patients with lymphoma/CLL with persistent or relapsing disease after RIC AlloSCT. From the first report in 1991 [15], GVLE has been explored as a therapeutic approach following AlloSCT, but the best approach to clinical management of patients relapsing after AlloSCT is yet to be established. Herein we describe therapeutic approaches adopted in these patients and analyze their outcome, focusing on patients with clinically demonstrable GVLE.
In our series, the disease response of the 19 patients who had been managed with an immune-based approach was associated with the development of GVHD, and no patients who did not develop GVHD responded to the disease. Although the series does not comprise many patients, our study indicates GVHD and GVLE to be complementary phenomena following AlloSCT in lymphoid malignancies. The best results were associated with the development of severe cGVHD, so clinicians should consider it as a potential curative approach, at least for those patients with persistent disease or for those who relapse after transplant. Moreover, on the basis of our experience, withdrawal of IST and/or DLI should be attempted in these patients. Although GVHD toxicity is a serious matter, only one death was ascribed to cGVHD and no death was associated with a GVHD; moreover, all patients who still presented active GVHD at the final follow-up had good quality of life and acceptable performance status (ECOG = 1). More importantly, we were able to induce long-term remissions in patients with very poor prognosis. If we consider the group of 11 patients who achieved CR after GVLE, they maintained CR, achieving a plateau for an estimated 4-year PFS of 40%.These results are even better in patients with severe GVHD (4-year PFS of 51%). These survival data are similar to those from the whole series of 112 AlloSCTs in lymphoid malignancies in our center (55.4% OS and 48.2% PFS). It is also important to point out that none of these patients had previously developed acute GVHD more severe than grade II, which could be associated with a higher risk of relapse.
When we analyzed the data paying attention to the specific diagnosis, GVLE appeared to be present in all histologies, but its influence was slight in HL compared with NHL and CLL. These results are consistent with other published data in relapsed HL after AlloSCT [16]. In a recent multicenter study from a cooperative Spanish-British prospective clinical trial, the overall response rate after DLI was 40%, but no patient sustained their response over the long-term [17]. Based on this low sensitivity to GVLE in HL, future clinical trials of such patients should test alternative strategies, such as the combination of the immune approach with new drugs, as has been recently done with promising results [18]. By contrast, in patients with NHL and CLL, a high response rate was recorded, including 7/11 patients (63.6%) who experienced long-term CR.
In the case of aggressive NHL (TCL and DLBCL), we observed 4/4 responses after GVLE enhancement. Management of post-AlloSCT relapses in aggressive NHL remains unexplored, and individualized approaches should be considered. Our study supports GVLE enhancement in this type of NHL in line with other reports [3,8,19].
It is well known that indolent lymphomas usually respond to IST withdrawal or DLI, with favorable responses in 77% of FLs [20,21] and around 60% of CLLs [22,23]. Results in our series confirm these findings, since 78% of our CLL and indolent lymphomas responded to this strategy.
In summary, according to our results, we can recommend an immune-based approach for treating persistent disease or relapse after AlloSCT because it appears to yield better responses than those arising from standard management approaches. In other words, allogenic transplantation should be seen as a continuous treatment in which relapses do not necessarily imply failure. However, data are still heterogeneous and results vary depending on diagnosis, which means that response to GVLE relies on a mechanism that remains to be elucidated. Efforts towards understanding biological aspects of GVLE have to be made in order to understand how to apply targeted therapy for improving response while minimizing toxicity.
References
- Caballero D, García-Marco JA, Martino R, Mateos V, Ribera JM, Sarrá J, et al. Allogeneic transplant with reduced intensity conditioning regimens may overcome the poor prognosis of B-cell chronic lymphocytic leukemia with unmutated immunoglobulin variable heavy-chain gene and chromosomal abnormalities (11q- and 17p-).Clin Cancer Res. 2005; 11: 7757-7763.
- Richardson SE, Khan I, Rawstron A, Sudak J, Edwards N, Verfuerth S, et al. Risk-stratified adoptive cellular therapy following allogeneic hematopoietic stem cell transplantation for advanced chronic lymphocytic leukaemia. Br J Haematol. 2013; 160: 640-648.
- Itonaga H, Tsushima H, Taguchi J, Fukushima T, Taniguchi H, Sato S, et al. Treatment of relapsed adult T-cell leukemia/lymphoma after allogeneic hematopoietic stem cell transplantation: the Nagasaki Transplant Group experience. Blood. 2013; 121: 219-225.
- Piñana JL, Martino R, Gayoso J, Sureda A, de la Serna J, Díez-Martín JL, et al. Reduced intensity conditioning HLA identical sibling donor allogeneic stem cell transplantation for patients with follicular lymphoma: long-term follow-up from two prospective multicenter trials. Haematologica. 2010; 95: 1176-1182.
- Khouri IF, Bassett R, Poindexter N, O'Brien S, Bueso-Ramos CE, Hsu Y, et al. Nonmyeloablative allogeneic stem cell transplantation in relapsed/refractory chronic lymphocytic leukemia: long-term follow-up, prognostic factors, and effect of human leukocyte histocompatibility antigen subtype on outcome. Cancer. 2011; 117: 4679-4688.
- Alvarez I, Sureda A, Caballero MD, Urbano-Ispizua A, Ribera JM, Canales M, et al. Nonmyeloablative stem cell transplantation is an effective therapy for refractory or relapsed Hodgkin lymphoma: results of a Spanish prospective cooperative protocol. Biol Blood Marrow Transplant. 2006; 12: 172-183.
- Cruz JG, Martino R, Balsalobre P, Inmaculada Heras, Josè Luis Piñana, David Serrano, et al. Long-term results of Fludarabine/Melphalan as a reduced-intensity conditioning regimen in mantle cell lymphoma: the GELTAMO experience. Ther Adv Hematol. 2011; 2: 5-10.
- NH Russell, JL Byrne, RD Faulkner, Gilyead M, Das-Gupta EP, Haynes AP. Donor lymphocyte infusions can result in sustained remissions in patients with residual or relapsed lymphoid malignancy following allogeneic haemopoietic stem cell transplantation. BoneMarrow Transplant. 2005; 36: 437-441.
- Dreger P, Corradini P, Kimby E, Michallet M, Milligan D, Schetelig J, et al. Indications for allogeneic stem cell transplantation in chronic lymphocytic leukemia: the EBMT transplant consensus. Chronic Leukemia Working Party of the EBMT. Leukemia. 2007; 21: 12-17.
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphoma: NCI Sponsored International Working Group. J Clin Oncol. 1999; 17: 1244-1253.
- Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A matched sibling donors. Transplantation. 1974; 18: 295–304.
- Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005; 11: 945-956.
- Cabrero M, Martín A, Gayoso J, et al. Phase II study of yttrium-90-ibritumomab tiuxetan in reduced intensity conditioning (RIC) allogeneic transplant in relapsed or refractory aggressive B-cell lymphoma: a GELTAMO phase II clinical trial. 16th Congress of European Hematology Association. Haematologica. 2011; 96: 413-414.
- Perez-Simóón JA, Martino R, Parody R, Cabrero M, Lopez-Corral L, Valcarcel D, et al. The combination of sirolimus plus tacrolimus improves outcome after reduced-intensity conditioning, unrelated donor hematopoietic stem cell transplantation compared with cyclosporine plus mycofenolate. Haematologica. 2013; 98: 526-532.
- Jones RJ, Ambider RF, Piantadosi S, Santos GW. Evidence of a graft-versus-lymphoma effect associated with allogeneic bone marrow transplantation. Blood. 1991; 77: 649-653.
- Anderlini P, Acholonu SA, Okoroji GJ, Andersson BS, Couriel DR, De Lima MJ, et al. Donor leukocyte infusions in relapsed Hodgkin's lymphoma following allogenic stem cell transplantation: CD3+ cell dose, GVHD and disease response. Bone Marrow Transplant. 2004; 34: 511-514.
- Sureda A, Canals C, Arranz R, Caballero D, Ribera JM, Brune M, et al. Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory Hodgkin’s lymphoma. Results of the HDR-ALLO study – a prospective clinical trial by the Grupo Español de Linfomas/ Trasplante de Médula Osea (GEL/TAMO) and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2012; 97: 310-317.
- Theurich S, Malcher J, Wennhold K, Shimabukuro-Vornhagen A, Chemnitz J, Holtick U, et al. Brentuximab vedotin combined with donor lymphocyte infusions for early relapse of Hodgkin lymphoma after allogeneic stem-cell transplantation induces tumor-specific immunity and sustained clinical remission. J Clin Oncol. 2013; 31: 59-63.
- Bishop MR, Dean RM, Steinberg SM, Odom J, Pavletic SZ, Chow C, et al. Clinical evidence of a graft-versus-lymphoma effect against relapsed diffuse large B-cell lymphoma after allogeneic hematopoietic stem-cell transplantation. Ann Oncol. 2008; 19: 1935-1940.
- Thomson KJ, Morris EC, Milligan D, Parker AN, Hunter AE, Cook G, et al. T-cell-depleted reduced-intensity transplantation followed by donor leukocyte infusions to promote graft-versus-lymphoma activity results in excellent long-term survival in patients with multiply relapsed follicular lymphoma. J Clin Oncol. 2010; 28: 3695-3700.
- Bloor AJ, Thomson K, Chowdhry N, Verfuerth S, Ings SJ, Chakraverty R, et al. High response rate to donor lymphocyte infusion after allogeneic stem cell transplantation for indolent non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008; 14: 50-58.
- Khouri I, Bassett R, Poindexter N, O'Brien S, Bueso-Ramos CE, Hsu Y, et al. Nonmyeloablative allogeneic stem cell transplantation in relapsed/refractory chronic lymphocytic leukemia. Cancer. 2011; 117: 4679-4688.
- Richardson SE, Khan I, Rawstron A, Sudak J, Edwards N, Verfuerth S, et al. Risk-stratified adoptive cellular therapy following allogeneic hematopoietic stem cell transplantation for advanced chronic lymphocytic leukaemia. Br J Haematol. 2013; 160: 640-648.