
Research Article
Ann Hematol Onco. 2023; 10(6): 1442.
Cytogenetic and Molecular Remission in Chronic Myeloid Leukemia in Togo
Padaro E¹; Womey KMC¹*; Layibo Y²; Kueviakoe MDI²; Magnang H¹; Mawussi K³; Agbetiafa K¹; Agbado KS¹; Vovor A²
¹Department of Hematology, University of Lomé, Togo
²Laboratory of Hematology, University of Lomé, Togo
³Kara Regional Blood Transfusion Center, University of Kara, Togo
*Corresponding author: Womey KMC Department of hematology, University of Lomé, 01BP1515, Togo. Tel: +22892526400 Email: corcellar.womey@gmail.com
Received: October 09, 2023 Accepted: November 13, 2023 Published: November 20, 2023
Abstract
The availability of tyrosine kinase inhibitors (TKIs) in Togo through the GIPAP program has revolutionized the management of chronic myeloid leukemia and increased patient life expectancy. However, diagnosis and molecular monitoring of therapeutic efficacy remain a real challenge due to the inaccessibility of cytogenetic and molecular biology tools. We’ve conducted, a cross-sectional, descriptive study that ran from December 21, 2022, to January 20, 2023, with the aim to evaluate the cytogenetic and molecular remission in CML patients followed at the CHU Campus de Lomé in Togo. Patients diagnosed with chronic-phase CML who had been treated for at least three months with imatinib or dasatinib and had achieved complete hematological remission were included. Dosage of BCR-ABL transcript levels was performed by RQ-PCR in Seattle, USA, using Dried Blood Spot. The transcript detection limit was 0.003% i.e., MR4. A total of 38 patients were included, 68.4% of them were treated with imatinib and 31.6% with dasatinib. In terms of remission, 28.9% had not achieve a partial cytogenetic remission while 15.8% of patients had achieved a MR4 remission and 7.9% a major molecular remission. Major molecular remission was achieved at a mean follow-up time of 95 months for patients taking imatinib and 22 months for those taking dasatinib. Patients in MR4 were on imatinib after a mean of 68 months. All in one, our study showed a high rate of treatment failure. Better access to cytogenetic and molecular biology tools is needed to improve management.
Keywords: CML; TKIs; remission; cytogenetic; molecular biology; Togo
Abbreviations: TKI; GIPAP; CML; RQ-PCR; CHR; MR; MMR; PCyR; CCyR, NR; DBS; ELN; CHR
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative syndrom resulting from the translocation t(9;22)(q34;q11) which gives rise to the BCR-ABL1 fusion gene encoding a protein with exacerbated tyrosine kinase function [1]. Previously always fatal, CML has become a chronic disease with a life expectancy close to that of the general population, thanks to the discovery of tyrosine kinase inhibitors (TKIs) [2]. International guidelines based on the quantification of BCR-ABL1 mRNA by real-time RT-PCR enable clinicians to regularly assess therapeutic efficacy [3]. In Togo in 2006, according to a study by Kueviakoe et al, CML accounted for 20% of hematological malignancies, with an annual hospital incidence rate of 2.91 cases [4]. This figure is constantly rising. Since the advent of imatinib in 2005, courtesy of The Max Foundation's GIPAP (Glivec International Patient Assistance Program), patient outcomes have improved considerably [5]. The range of TKIs offered by the GIPAP program was subsequently extended with the introduction of dasatinib in 2019 and bosutinib in 2023. However, molecular monitoring of patients is a real challenge because of the difficulty of accessing cytogenetic and molecular tests. The aim of this study, conducted with the support of The Max Foundation, was therefore to investigate the molecular remission to TKIs in patients treated for chronic myeloid leukemia at the CHU Campus in Lomé, Togo.
Materials and Methods
This was a cross-sectional, descriptive study, which took place from 21 December 2022 to 20 January 2023. It concerned patients followed up in the clinical hematology department of the CHU Campus and who were seen in consultation during the study period. Patients diagnosed with chronic-phase CML who had been treated for at least three months with imatinib or dasatinib and had achieved complete hematological remission were included, within the limits of available DBS (Dried Blood Spot) samples. Patients diagnosed during the study period and those with other hematological malignancies were not included. The variables studied were age, sex, length of follow-up, type of TKI received and BCR-ABL molecular transcript level. Cytogenetic and molecular response was assessed according to the International Scale as the ratio of BCR-ABL1 transcripts to ABL1 transcripts and was expressed and reported as BCR-ABL1 % on a log scale. BCR_ABL =10% is equivalent to partial cytogenetic remission (PCyR). BCR-ABL1 transcript level =1% is defined as complete cytogenetic remission (CCyR). BCR-ABL1 transcript level =0.1% is defined as major molecular response (MMR) or MR3. A BCR-ABL1 transcript level =0.01% or undetectable disease in cDNA with >10,000 ABL1 transcripts is defined as MR4 [6]. The BCR-ABL transcript level was measured in Seattle, USA, by RQ-PCR using Dried Blood Spot (DBS). The transcript detection limit was 0.003% i.e MR4.
Results
Thirty-eight patients were included in our study, with a mean age of 42 ± 16 years (8-75) and a sex ratio of 1.4:1. Twenty-six (68.4%) patients were treated with imatinib and 12 (31.6%) with dasatinib. The mean duration of follow-up was 51±55 months (7-211). It was 62 months for patients taking imatinib and 27±15 months for patients taking dasatinib. Overall, 28.9% of patients had no molecular remission, compared with 15.8% who had achieved a MR4 (Figure 1). Depending on the treatment, a MMR was observed in 8.3% of patients taking dasatinib, compared with 7.7% of patients taking imatinib. Table I shows the different types of molecular remission according to TKI. On imatinib, patients on MMR had a mean follow-up of 95 months, compared with 22 months for patients on dasatinib. Tables II and III show the different types of remission obtained according to treatment and duration of follow-up.
Molecular remission, n(%)
Dasatinib
Imatinib
Total
(n=12)
(n=26)
(N=38)
NR
6(50,0)
5(19,2)
11(28,9)
PCyR
4(33,3)
9(34,6)
13(34,2)
CCyR
1(8,3)
4(15,4)
5(13,2)
MMR
1(8,3)
2(7,7)
3(7,9)
MR4
0(0,0)
6(23,1)
6(15,8)
Table 1: Molecular remission by TKI.
Follow-up time (months)
NR
PCyR
CCyR
MMR
MR4
Total
(n=5)
(n=9)
(n=4)
(n=2)
(n=6)
(n=26)
Median [IIQ]
69(23-69)
22(9-55)
27(21-75)
95(60-131)
49(22-110)
29(21-72)
Mean(±SD)
51(26)
53(68)
70(95)
95(100)
68(59)
62(62)
Mini-Maxi
22-73
7-205
14-211
24-166
14-154
7-211
Table 2: Length of follow-up for patients on imatinib by type of molecular remission.
Follow-up time (months)
NR
PCyR
CCyR
MMR
MR4
Total
(n=6)
(n=4)
(n=1)
(n=1)
(n=0)
(N=12)
Median [IIQ]
25(16-29)
28(19-44)
29(29-29)
22(22-22)
-
25(19-29)
Mean(±SD)
22(8)
35(24)
29(NA)
22(NA)
-
27(15)
Mini-Maxi
11-29
14-69
29-29
22-22
-
11-69
Table 3: Length of follow-up for patients on dasatinib by type of molecular remission.
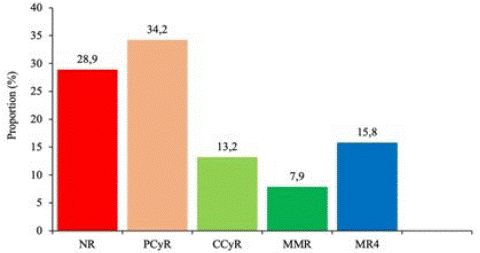
Figure 1: Overall distribution of patients according to type of remission.
Discussion
CML is a frequent malignant disease in our practice [4] and its prevalence is constantly increasing due to the spectacular increase in patients' life expectancy, as a result of the systematic use of TKIs. Although the efficacy of TKIs no longer needs to be demonstrated, access to them in developing countries such as Togo is difficult, and has only been possible for the majority of patients thanks to the support of the GIPAP program [7].
Diagnosis and monitoring of CML are based on cytogenetics, combining conventional cytogenetics with molecular analysis using RQ-PCR to quantify BCR-ABL transcripts [7]. These molecular techniques are now essential for the diagnosis and prognosis of CML. However, because of the difficulty of accessing these tests in our practice, the diagnosis of CML is still based on cytological criteria (hemogram and myelogram), and follow-up is based solely on monitoring the achievement and maintenance of a complete hematological remission (CHR). Our study therefore enabled us to evaluate the molecular remission in patients followed for CML and in CHR. Its main limitations are its cross-sectional nature, which does not allow us to establish the actual mean time taken to obtain the various molecular remissions, and the small sample size due to the number of cards available for DBS sampling.
CML is a disease of the young, as demonstrated by the results of our study, which are consistent with those in the literature [8,9]. We found a slight male predominance, as did Koffi et al. in Côte d'Ivoire [9].
Overall, almost 30% of patients in our study had no partial cytogenetic remission after three months of follow-up. In addition, the mean time taken to obtain the different types of molecular remission, whether with imatinib or dasatinib, was well above the standards required according to the ELN recommendations, suggesting a high percentage of patients in therapeutic failure. The ELN defines treatment failure as the absence of partial cytogenetic remission (BCR-ABL1 >10%) between 1 and 3 months, complete cytogenetic remission (BCR-ABL1 >10%) after 6 months, major molecular remission (BCR-ABL1 >1%) after 12 months and loss of MMR or the occurrence of resistance or additional high-risk cytogenetic abnormalities [6]. This high percentage of failure in our study could be explained by late diagnosis of the disease, poor prognosis with a high Sokal score at diagnosis, poor therapeutic compliance, but also by bad indication of TKIs. In our practice, all newly diagnosed CML patients are started on imatinib as first-line therapy, while other treatment lines (dasatinib and bosutinib) are started in the event of resistance or intolerance. This indication, which does not correspond to international recommendations, is linked to the fact that imatinib was for a long time the only TKI available. The arrival of other TKIs could have improved prescribing, but the difficulty of performing caryotyping and molecular biology continues to hamper good practice. Indeed, when choosing a first-line TKI in the chronic phase of CML, it is necessary to take into account the prognosis, as well as additional cytogenetic abnormalities [10]. It is important to note that resistance to imatinib has rapidly emerged [3]. Apart from poor compliance, this resistance will depend either on the molecule itself (pharmacokinetics, influx and efflux pumps), on the leukemic cell due to genetic instability or activation of other oncogenic signalling pathways, or on the target of the TKI (gene amplification, kinase domain mutation) [3]. Over 100 mutations affecting more than 70 amino acids have been identified [11,12]. They are associated with reduction in sensitivity to imatinib [13]. Four other TKIs are currently approved for first-line treatment in CML: dasatinib, nilotinib, bosutinib and radotinib [14-16]. Although there are no comparative studies, the choice of TKI should be guided by the sensitivity profile of specific BCR-ABL mutations if possible. Indeed, in cases of T315I mutation, only ponatinib, a 3rd generation TKI, is effective [17-20]. F317L/V/I/C and T315A mutations warrant treatment with nilotinib, bosutinib or ponatinib, whereas the V299L mutation indicates treatment with nilotinib or ponatinib [6]. The therapeutic indications are therefore not optimal in our daily practice and explain this high rate of therapeutic failure.
Even so, our study found 15.8% of patients in MR4, all of them on imatinib. This is explained by the length of follow-up and the use of imatinib as first-line treatment. However, the possibility of discontinuing treatment, as envisaged in the STIM protocol [21], should not be considered in these patients.
Conclusion
Despite considerable progress in the management of CML, and the availability of TKIs through the GIPAP program, diagnosis and optimal follow-up remain a real challenge in resource-limited countries such as Togo. Our study revealed a high rate of therapeutic failure in patients in complete hematological remission. This highlights the urgent need to make cytogenetic and molecular diagnostic techniques more accessible, with the aim of improving the indication of first line TKIs, as well as ensuring appropriate therapeutic change in the event of resistance or intolerance.
References
- Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 5th ed. 2022; 36: 1703-19.
- Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM-L. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016; 34: 2851-7.
- Sorel N, Cayssials é, Brizard F, Chomel JC. Actualisation des traitements et du suivi moléculaire dans la prise en charge de la leucémie myéloïde chronique. Ann Biol Clin (Paris). 2017; 75: 129-45.
- Kueviakoe IM, Baite K, Agbetiafa K, Vovor A, Segbena AY. Panorama des hémopathies malignes diagnostiquées au myélogramme dans les CHU de Lomé sur une période de 12 ans. Rev Togol Sci. 2006; 1: 57-68.
- Kueviakoe MI, Agbetiafa K, Padaro E, Layibo Y, Kolou M, Amavi T, et al. Profil évolutif des Patients Souffrant de Leucémie Myéloïde Chronique Sous imatinib au Togo: étude de 63 Cas Colliges en 10 ans au Chu-Campus de Lome. J Rech sci l’université Lomé. 2014; 16: 473-82.
- Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020; 34: 966-84.
- Koffi G. Leucémie myéloïde chronique en Afrique subsaharienne: accès à la biologie moléculaire et aux inhibiteurs de tyrosine kinase, Expérience de la Côte d’Ivoire. Corresp Onco-Hematol. 2023; XVIII: 27-30.
- Höglund M, Sandin F, Simonsson B. Epidemiology of chronic myeloid leukaemia: an update. Ann Hematol. 2015; 94: S241-7.
- Koffi KG, Nanho DC, N’dathz E, Kouehion P, Dissieka R, Attia A, et al. The effect of imatinib for newly diagnosed philadelphia chromosome-positive, chronic-phase myeloid leukemia in sub-Saharan african patients: the experience of côte d’ivoire. Adv Hematol. 2010; 2010: 268921.
- Fabarius A, Leitner A, Hochhaus A, Müller MC, Hanfstein B, Haferlach C, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011; 118: 6760-8.
- Ernst T, Hoffmann J, Erben P, Hanfstein B, Leitner A, Hehlmann R, et al. ABL single nucleotide polymorphisms may masquerade as BCR-ABL mutations associated with resistance to tyrosine kinase inhibitors in patients with chronic myeloid leukemia. Haematologica. 2008; 93: 1389-93.
- Reddy EP, Aggarwal AK. The ins and outs of bcr-abl inhibition. Genes Cancer. 2012; 3: 447-54.
- O’Hare T, Eide CA, Deininger MW. Bcr-abl kinase domain mutations, drug resistance, and the road to a cure for chronic myeloid leukemia. Blood J Am Soc Hematol.. Blood. 2007; 110: 2242-9.
- Guilhot J, Preudhomme C, Mahon FX, Guilhot F. Analyzing molecular remission in chronic myeloid leukemia clinical trials: pitfalls and golden rules. Cancer. 2015; 121: 490-7.
- Kwak JY, Kim SH, Oh SJ, Zang DY, Kim H, Kim JA, et al. Phase III clinical trial (RERISE study) results of efficacy and safety of radotinib compared with imatinib in newly diagnosed chronic phase chronic myeloid leukemia. Clin Cancer Res. 2017; 23: 7180-8.
- Do YR, Kwak JY, Kim JA, Kim HJ, Chung JS, Shin HJ, et al. Long-term data from a phase 3 study of radotinib versus imatinib in patients with newly diagnosed, chronic myeloid leukaemia in the chronic phase (RERISE). Br J Haematol. 2020; 189: 303-12.
- Cortes JE, Kim DW, Pinilla-Ibarz Jl, Le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in Philadelphia chromosome–positive leukemias. N Engl J Med. 2013; 369: 1783-96.
- Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, et al. Ponatinib efficacy and safety in Philadelphia chromosome–positive leukemia: final 5-year results of the phase 2 PACE trial Soc Hematol.. Blood. 2018; 132: 393-404.
- Lipton JH, Chuah C, Guerci-Bresler A, Rosti G, Simpson D, Assouline S, et al. Ponatinib versus imatinib for newly diagnosed chronic myeloid leukaemia: an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2016; 17: 612-21.
- Andrews C, Lipton J. The role of ponatinib in chronic myeloid leukemia in the era of treatment free remission. Leuk Lymphoma. 2019; 60: 3099-101.
- Rousselot P, Huguet F, Rea D, Legros L, Cayuela JM, Maarek O, et al. Imatinib discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007; 109: 58-60.