
Research Article
Ann Hematol Onco. 2023; 10(5): 1438.
Secondary Malignancies in Patients after Allogeneic Hematopoietic Cell Transplantation at Triple the Incidence of Malignancies in Their Family Donors
Santarone S1*; Angelini S2; Natale A1; Olioso P1; Vaddinelli D1; Spadano R1; Di Bartolomeo P1
¹Department of Oncology Hematology, Ospedale Civile, Pescara, Italy
²UOC Ematologia e Terapia Cellulare, Ospedale Mazzoni, Ascoli Piceno, Italy
*Corresponding author: Santarone S Department of Oncology Hematology, Ospedale Civile, Via Fonte Romana 8, Pescara 65125, Italy Tel +39 0854252689; Fax: +39 0854242583 Email: stella.santarone@virgilio.it
Received: August 17, 2023 Accepted: September 11, 2023 Published: September 18, 2023
DOI: https://doi.org/10.26420/annhematoloncol.2023.1438
Abstract
The primary end point of this retrospective study was to determine the incidence, risk factors and clinical outcome of secondary malignancies in 951 patients who were given an allogeneic Hemopoietic Cell Transplantation (HCT) and to compare them with the incidence of malignancy observed in the cohort of 761 stem cell family donors. With a median follow-up of 20 years, 74 HCT recipients (40 males) developed SM at a median of 16.09 years since transplant and at a median age of 47 years. The 35-yr cumulative incidence of SM was 17.0% (95% confidence interval, 12.8-21.6%). In univariate analysis, factors associated with increased incidence of SM were cumulative (limited and extensive) chronic graft-versus-host disease (cGvHD) and duration of cumulative cGvHD >24 months. By multivariate analysis, cumulative cGvHD was the only independent risk factor for SM. Patients with cGvHD had 2.85x higher risk as compared to patients without cGvHD (P<0.001). With a median follow-up of 18 years, 13 family donors (7 males) out of 761 developed malignancy at a median of 15.04 years since stem cell donation and at a median age of 55 years. As compared to the cumulative incidence of SM observed in the cohort of transplant recipients, the cumulative incidence of malignancy in family donors at 35 years since stem cell donation was statistically lower [5.8% vs 17.0% (P=0.001)]. This study demonstrates that HCT recipients have a significantly higher incidence of developing post-transplant malignancy as compared to family donors and that cGvHD is a strong risk factor for SM development.
Keywords: Malignancy; HCT; cGvHD; Stem cell donors
Introduction
Allogeneic Hematopoietic Cell Transplant (HCT) represents a potential curative procedure for a variety of malignant and nonmalignant hematologic disorders. Improvement in survival rate following HCT has resulted in a need to assess issues related to long-term complications, including the development of Secondary Malignancy (SM), which represent an important cause of late morbidity and mortality. Malignancies occurring after HCT fall into three general categories: hematologic malignant diseases, lymphoproliferative disorders, and solid tumors [1]. Several studies have reported that the magnitude of the increased risk of SM has ranged from 2.1- to 2.7-fold when compared to an age- and sex-matched general population and the risk among long-term survivors ranges from 3% to 15% at 15 years after transplantation [2-9]. We conducted a single center retrospective cohort study to determine the incidence, risk factors and clinical outcome of SM in patients who survived after allogeneic HCT and took into account the incidence of malignancies among their stem cell family donors.
Methods
Study Cohort
Our study included 989 consecutive patients who received an allogeneic HCT between March 1977 and December 2018 at the Bone Marrow Transplant Center of Pescara, Italy. Patients with Fanconi anemia (n=12), acquired immunodeficiency syndrome (n=2), and Down syndrome (n=2) were excluded because of their inherent susceptibility to cancer. Patients with a history of solid cancer before HCT (n=22) were also excluded. The study was approved by the local institutional review board. Informed consent for HCT and for follow-up studies was obtained from all patients and donors or their legal guardians in accordance with the Declaration of Helsinki.
Data
Data were extracted from the Allogeneic Transplant Program Database of the Center and included patient and donor demographic information (age, gender), diagnosis of hematologic disease, stem cell source, donor relationship and HLA compatibility, intensity of the conditioning regimen defined as Myeloablative (MAC) or Reduced-Intensity (RIC), drugs used in the preparative therapy, and drugs used as Graft-Versus-Host disease (GvHD) prophylaxis. Other information regarding the post-transplant clinical outcome including engraftment, acute GvHD (aGvHD) and chronic GvHD (cGvHD) occurrence, relapse of the original disease, survival and most important and common transplant-related complications (veno-occlusive disease of the liver, hemorrhagic cystitis, infections, neurological and cardiac involvement) were also obtained from the Database. History of duration and quantity of smoking, alcohol consumption and drug abuse before and after HCT were collected only for patients diagnosed with SM and for donors who developed a malignancy.
Transplant Procedure
Each recipient was given Unique Patient Number (UPN). The day of transplant was designated as day 0. The intensity of conditioning regimen, MAC vs RIC, was defined following the criteria published by the Center for International Blood and Marrow Transplantation Research [10]. RIC regimens were generally administered to patients over age 60 years or recipients with comorbidities that precluded the use of MAC. aGVHD was diagnosed according to Glucksberg’s criteria [11], and cGvHD according to the modified Seattle criteria (for categorization of cGVHD as clinical limited or clinical extensive) [12].
Post-Transplant Cancer Screening
In the first 5 years after transplantation, all patients were followed at least annually at the transplant Center and every 2 to 3 years later or when a new clinical event appeared. In any occasion patients were asked to give information on the clinical health status of their stem cell donors. Patients were informed about the importance of an accurate screening for common cancers using brochures illustrating the increased risk of SMs after allografts. Information on SM, including date of diagnosis, site of involvement, morphologic features, therapy (surgery alone, chemotherapy, radiotherapy, immunotherapy, palliation), and outcome with Eastern Cooperative Oncology Group (ECOG) performance score at last follow-up were collected by a review of medical records provided either by the patients or by the physician who had made the diagnosis and had taken care of them. The same procedure was followed for the donors who manifested the occurrence of any malignancy. Cancer type was classified according to International Classification of Diseases, 10th revision. Pathology and physician reports of each case of SM were reviewed centrally at the transplant center by a committee including the transplant expert, the pathologist, the surgeon expert in each type of tumor, and the oncologist.
Statistical Analysis
A descriptive analysis of all variables was performed including mean, median, standard deviation, range, minimum and maximum value for continuous variables, absolute and relative frequencies for categorical variables. Using parametric and nonparametric statistical procedures, the possible interdependence between 2 or more variables was evaluated and a P value of .05 was considered significant.
Taking into consideration death without occurrence of malignancy as competing risk, the probability of both SM in transplant recipients and malignancies in stem cell donors has been studied by fitting cumulative incidence function [13]. Univariate analysis of possible factors predicting for SM in transplant recipients included: age at transplant, gender, underlying hematologic disease, type of underlying hematologic disease, ferritin level at HCT, radiotherapy performed before transplantation, number and type of transplants, drugs used in conditioning therapy, intensity of conditioning regimen, use of anti-thymocyte globulin, GvHD prophylaxis, donor relationship and compatibility, stem cell source, occurrence and duration of cGvHD. The curves of various subgroups were compared using the Gray’s test whereas the duration of cGvHD was compared using the Mann-Whitney test [14]. The duration of cGvHD was transformed into categorical variable and the 24 months cut-off value was identified as follows: i) with graphic investigations using Martingale residual plots [15]; ii) with maximization of the Gray test; and iii) on the basis of medical expertise and consensus. The joint effect of variables on cumulative incidence function of SM was evaluated using the multivariate model of Fine and Gray were the occurrence of aGvHD and cGvHD was treated as a time-dependent covariate [15]. Covariates were selected in the multivariate analysis using a stepwise procedure adapted to multiple imputation methodology. The probability of Overall Survival (OS) and Disease-Free Survival (DFS) was calculated with the method of Kaplan-Meyer [16].
Statistical analyses were performed with the use of R Statistical Software (version 3.3.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
The final study population included 951 patients (532 males, 56%). Ninety-eight patients underwent a second allogeneic HCT for either primary or secondary graft failure (n=26) or leukemia relapse (n=72). Moreover, 104 patients had received an autologous HCT before allogeneic transplant as part of the therapeutic program. Baseline patient and donor clinical characteristics are shown in Table 1.
Patients
%
Number
951
Gender, male/female
532/419
56/44
Median age, yr (range)
33(1-71)
1-18 yr
259
27.2
19 – 50 yr
494
52
51-71 yr
198
20.8
Underlying disease
MD/NMD
773/178
81.3/18.7
Median ferritin, ng/mL (range)
910(5-14210)
<1000
510
53.6
1001–2000
296
31.2
>2000
145
15.2
Patients treated with radiotherapy before HCT
128*
13.5
DONORS
Number
951
Gender, male/female
550/401
57.8/42.2
Median age, yr (range)
32(0-70)
Relationship and compatibility
HLA identical sibling
638
67.1
Unrelated
190**
20
Haploidentical relative
123
12.9
TRANSPLANT
Intensity of conditioning
Myeloablative
788
82.9
Reduced intensity
163
17.1
Drugs used in conditioning regimen
TBI CY
217***
22.8
BU CY + FLU + TH
611****
64.2
TREO FLU
26
2.7
TH MEL FLU
31
3.3
TH FLU CY
35
3.7
CY FLU
31
3.3
GvHD prophylaxis
CSA
191
20.1
CSA MTX
639
67.2
CSA MTX MMF anti-CD25
84
8.8
T-cell depletion
37
3.9
ATG as part of GvHD prophylaxis
Yes
328
34.5
No
623
65.5
Stem cell source
BM
644
67.7
PBSC
302
31.8
CB
5
0.5
MD malignant disease (acute myeloid leukemia n=313; acute lymphoblastic leukemia n=168; chronic myeloid leukemia n=115; myelodisplastic syndrome n=64; multiple myeloma n=46; non-Hodgkin lymphoma n=21; Hodgkin lymphoma n=25; primary myelofibrosis n=21);
NMD nonmalignant disease (thalassemia major n=137; severe aplastic anemia n=31; paroxysmal nocturnal hemoglobinuria n=2; chronic granulomatous disease n=2; sickle cell disease n=4;combined immunologic deficiency n=1; hemophagocytic lymphohistiocytosis n=1); HCT hemopoietic cell transplantation; TBI total body irradiation; CY cyclophosphamide; BU busulfan; FLU fludarabine; TH thiotepa; TREOtreosulfan; MEL melfalan; GvHD graft-versus-host disease; CSA cyclosporine A; MTX methotrexate; MMFmycophenolate mofetil; ATG antithymocyte globulin; BM bone marrow; PBSC peripheral blood stem cells; CB cord blood.
* Includes patients affected by chronic myeloid leukemia who received 10 Gy splenic irradiation during conditioning regimen (n=45), patients affected by acute lymphoblastic leukemia who received 18-24 Gy central nervous system irradiation given as prophylaxis during first or second complete remission (n=43), patients affected by lymphoma who received 36-40 Gy nodal irradiation as part of their therapeutic protocol (n=18), patients affected by multiple myeloma who received 24-40 Gy irradiation of single or multiple bone lesions (n=19), patients affected by acute lymphoblastic leukemia who received 10 Gy testicular irradiation during conditioning regimen (n=3).
** Includes 10/10 HLA matched (n=31), 9/10 HLA matched (n=66), and 8/10 HLA matched donors (n=93).
*** Total body irradiation was given as a single total dose of 2 Gy (n=16), or a single total dose of 10 Gy (n=49), or a total dose of 12 Gy given in 6 fractionated doses over 3 days (n=152).
**** Busulfan was given either orally (n=348) or intravenously (n=263).
Table 1: Baseline Patient, Donor and Transplant Characteristics.
Transplantation
At time of data-censoring date (June 30, 2023), 458 patients out of 951 (48.2%) were living. The information on the health status of all these patients was updated in the last 3 months prior to study closure. Table 2 shows details of aGvHD and cGvHD occurrence, causes of death and survival. The median follow-up of survivors was 20 years (range, 4.2 to 41 years). The follow-up completeness index for the entire cohort of living recipients was 99% at 35 years after transplantation.
N.(%)
Patients
951
Not evaluable
39(4.1)
Evaluable
912(95.9)
No evidence of aGvHD
537(58.9)
aGvHD grade I-IV
375(41.1)
Not evaluable
145(15.2)
Evaluable
806(84.8)
No evidence of cGvHD
609(75.6)
cGvHD limited or extensive
197(24.4)
median duration cGvHD, months (range)
28(3-302)
cGvHD duration <24 months
80(40.6)
cGvHD duration >24 months
117(59.4)
Dead
493(51.8)
Due to transplant-related causes, (%)
201(21.1)
Due to relapse of original disease, (%)
207(21.8)
Due to secondary malignancy, (%)
25(2.6)
Due to any other cause, (%)
60(6.3)
Living
458(48.2)
Median follow-up, years (range)
20(4.2-41)
aGvHD acute graft-versus-host disease; cGvHD chronic graft-versus-host disease.
Table 2: Clinical outcome of acute and chronic GvHD, causes of death and survival.
Incidence and Outcome of SM in HCT Recipients
Seventy-four patients (40 males, 54%) were diagnosed with SM between July 1995 and March 2023 at a median of 16.09 years (range, 0.4 to 36.11 years) after HCT. We included all type of SM with the exclusion of Epstein-Barr virus-related lymphoproliferative disease and non-melanoma skin cancers. Six patients developed an additional second new SM at different time from the first malignancy (melanoma and prostate carcinoma in 1, carcinoma of the uterus and non-hodgkin lymphoma in 1, oral cavity carcinoma and lung carcinoma in 1, oral cavity carcinoma and esophagus carcinoma in 1, pancreas carcinoma and breast carcinoma in 1, oral cavity carcinoma and pharynx carcinoma in 1). One patient developed three different types of SM (oral cavity carcinoma, renal carcinoma and melanoma). The distribution of the first SM among patients affected by hematological nonmalignant and malignant disease and by type of underlying hematological disease is shown in Table 3. Type of SM, therapy (surgery, chemotherapy, radiotherapy, and palliation) and outcome are described in Table 4. Carcinoma of the oral cavity (n=15) was the most common type of SM followed by breast carcinoma (n=10), colon carcinoma (n=9), lung carcinoma (n=6), melanoma (n=6), carcinoma of uterus (n=5), and thyroid carcinoma (n=5). Thalassemia major was the disease with the highest incidence of SM in relation to the number of patients (17 cases of SM in 137 patients, 12.4%). The median age of patients with SM was 29.7 years (range, 2 to 67.09 years) and 47 years (range, 14 to 76.66 years) at HCT and at time of SM diagnosis, respectively. No patient had active cGvHD and none was receiving systemic immunosuppressive therapy at time of SM occurrence.
Primary diagnosis
N. of
patientsN. of
SMs%
Type of secondary malignancy
NMD
178
20
11.2
Thalassemia major
137
17
12.4
Oral cavity 5, Thyroid 4, Colon 2, Breast 1, Melanoma 1,
Merkel carcinoma 1, Parotid 1, Uterus 1, Liver 1Severe aplastic anemia
31
3
9.7
Oral cavity 2, Melanoma 1
Other
10
0
0
MD
773
54
7.0
AML
313
22
7.0
Breast 5, Oral cavity 3, Pancreas 3, Lung 3, Uterus 2, Colon 1,
Esophagus 1, Thyroid 1, Bladder 1, Brain 1, Kaposi sarcoma 1ALL
168
8
4.8
Colon 2, Oral cavity 1, Stomach 1, Breast 1, Liver 1, Brain 1, Melanoma 1
CML
115
14
12.2
Oral cavity 3, Colon 3, Larynx 1, Breast 1, Lung 1, Prostate 1, Uterus 1, Melanoma 1, NHL 1, Kidney 1
MDS
64
5
7.8
Lung 2, Melanoma 2, Colon 1
Lymphoma
46
3
6.5
Breast 1, Uterus 1, Oral cavity 1
MM
46
0
0
MF
21
2
9.5
Breast 1, HL 1
Total
951
74
7,8
NMD: Nonmalignant Disease; MD: Malignant Disease; AML: Acute Myeloid Leukemia; ALL: Acute Lymphoblastic Leukemia; CML: Chronic Myeloid Leukemia; MDS: Myelodisplastic Syndrome; HL: Hodgkin Lymphoma; NHL: Non-Hodgkin Lymphoma; MM: Multiple Myeloma; MF: Myelofibrosis
Table 3: Distribution of secondary malignancies by type of underlying disease.
The cumulative incidence of SM for the entire cohort of transplant recipients is depicted in Figure 1A. It amounts to 2.6% (95% confidence interval [CI], 1.7-3.9%) at 10 years, 5.6% (95% CI, 4.4-8.0%) at 20 years, 11.9% (95% CI, 9.2-15.0%) at 30 years, and 17.0% (95% CI, 12.8-21.6%) at 35 years post-HCT.
Type of SM
No.
Surgery
CH
RT
Palliation
Alive
Dead
First cause of death
Recipients
Oral cavity
15
8
4
8
10
5
SM 5
Breast
10
8
6
6
7
3
SM 3
Colon
9
8
8
2
1
7
2
SM 2
Lung
6
1
5
1
0
6
SM 6
Melanoma
6
6
5
1
pulmonary fibrosis 1
Uterus
5
2
2
1
3
2
SM 1, encephalopathy 1
Thyroid
5
5
2
4
1
leukemia relapse 1
Pancreas
3
3
3
0
3
SM 3
Brain
2
2
1
2
0
2
SM 1, brain abscess 1
Liver
2
2
1
1
1
1
SM 1
Kidney
1
1
1
0
1
SM 1
Esophagus
1
1
1
1
0
1
gastroenteric hemorrhage 1
Stomach
1
1
0
1
SM 1
Larynx
1
1
1
1
0
Parotid
1
1
1
0
1
SM 1
Prostate
1
1
1
0
Bladder
1
1
1
0
Merkel carcinoma
1
1
0
1
SM 1
Kaposi sarcoma
1
1
0
1
cGvHD
HL
1
1
1
0
NHL
1
1
0
1
pulmonary fibrosis 1
Total
74
51
38
23
3
41
33
SM 26, other causes 7
Donors
Breast
3
3
3
2
2
1
malignancy 1
Stomach
2
2
1
1
1
malignancy 1
Colon
2
2
1
1
0
2
malignancy 2
NHL
2
2
1
1
malignancy 1
Prostate
1
1
1
1
0
1
malignancy 1
Bladder
1
1
0
1
malignancy 1
Esophagus
1
1
1
0
1
malignancy 1
ALL
1
1
1
0
Total
13
10
10
4
5
8
malignancy 8
SM: Secondary Malignancy; CH: Chemotherapy; RT: Radiotherapy; cGvHD: Chronic Graft-Versus-Host Disease; HL: Hodgkin Lymphoma; NHL: Non-Hodgkin Lymphoma; ALL: Acute Lymphoblastic Leukemia
Table 4: Type of malignancy, therapy and outcome in recipients and donors.
As determined by univariate analysis (Table 5), the only factors associated with increased cumulative incidence of SM were occurrence of cumulative (limited and extensive) cGvHD (no vs yes, 16.3% [95% CI, 11.3-22.2%] vs 32.2% [95% CI, 19.5-45.6%], P<0.001), and duration of cumulative cGvHD (<24 months vs >24 months, 25.7% [95% CI, 10.9-43.5%] vs 38.0% [95% CI, 17.9-58.1%] P=0.018).
On multivariate analysis (Table 5), occurrence of cumulative cGvHD was the only independent risk factor associated with a higher rate of SM development. Recipients who were diagnosed with cumulative cGvHD had 2.85x higher than expected rate of SM (95% CI, 1.79-4.54%) (P<0.001). In an effort to minimize the effect of time, we included time period of HCT in the multivariate analysis, but this analysis did not demonstrate a significant effect on SM occurence.
No.
No. of events
Univariate analysis
Multivariate analysis
35-year cumulative incidence (95% CI)
P-value
HR (95% CI)
P-value
All patients
951
74
17.0% (12.8-21.6%)
Age
1-18 years
258
24
19.5% (12.4-27.8%)
19-50 years
495
39
15.4% (10.0-21.8%)
51-71 years
198
11
9.2% (4.4-16.2%) *
0.069
Gender
Male
532
40
15.5% (10.5-21.5%)
Female
419
34
18.6% (12.2-26.2%)
0.596
Underlying disease
MD
773
54
17.2% (12.2-22.9%)
NMD
178
20
20.9% (11.8-31.6%)
0.958
Hematologic disease
Acute leukemia (AML+ALL)
481
30
17.8% (11.2-25.7%)
CML
115
14
17.5% (9.5-27.5%)
Other MD
177
10
19.0% (4.5-59.5%)
Thalassemia
137
17
21.7% (10.9-34.9%)
SAA
31
3
20.3% (4.2-45.0%)
Other NMD
10
0
0%
0.913
Ferritin level at HCT
<1000 ng/mL
510
44
18.5% (13.1-24.6%)
1001-2000 ng/mL
296
20
10.7% (6.4-16.1%)
>2000 ng/mL
145
10
21.0% (7.5-38.9%)
0.166
Radiotherapy before HCT
No
823
63
16.4% (12.0-21.3%)
Yes
128
11
19.4% (9.1-32.7%)
0.956
Previous HCT
Single allo transplant
749
61
17.9% (13.0-23.4%)
Double allo transplant
98
9
16.6% (7.9-28.1%)
Double transplant auto + allo
104
4
4.9% (1.6-11.3%)
0.933
Drugs used in conditioning regimen
TBI CY
217
17
15.7% (9.5-23.1%)
Other
734
57
16.2% (11.2-22.0%)
0.446
Intensity of conditioning
MAC
788
63
17.3% (12.7-22.6%)
RIC
163
11
14.2% (5.9-26.0%)
0.565
ATG
No
623
61
17.7% (13.1-22.9%)
Yes
328
13
7.8% (3.7-14.0%)
0.088
GvHD prophylaxis
CSA
191
17
13.3% (7.9-20.1%)
CSA + MTX
637
53
25.7% (10.5-44.1%)
CSA + MTX + MMF + anti-CD25
84
3
6.7% (1.6-17.4%)
T-cell depletion
37
1
6.0% (0.3-26.3%)
0.143
Donor relationship / compatibility
HLA identical sibling
638
62
18.4% (13.6-23.7%)
Unrelated (any compatibility)
190
8
7.5% (2.6-15.7%) *
Haploidentical related
123
4
5.9% (1.8-13.8%) *
0.468
Stem cell source
BM
644
58
18.0% (13.3-23.3%)
PBSC
302
16
9.9% (4.7-17.3%)
CB
5
0
0%
0.111
cGvHD
Not evaluable
145
0
0
No
609
44
16.3% (11.3-22.2%)
1 (reference)
Yes (any extension)
197
30
32.2% (19.5-45.6%)
<0.001
2.95 (1.79-4.54)
<0.001
Duration of cGvHD
<24 months
80
7
25.7% (10.9-43.5%)
>24 months
117
23
38.0% (17.9-58.1%)
0.018
SM: Secondary Malignancy; CI: Confidence Interval; HR: Hazard Risk; MD: Malignant Disease; NMD: Nonmalignant Disease; AML: Acute Myeloid Leukemia; ALL: Acute Lymphoblastic Leukemia; CML: Chronic Myeloid Leukemia; SAA: Severe Aplastic Anemia; HCT: Hemopoietic Cell Transplantation; TBI: Total Body Irradiation; CY: Cyclophosphamide; MAC: Myeloablative Conditioning; RIC: Reduced-Intensity Conditioning; ATG: Antithymocyte Globulin; CSA: Cyclosporine; MTX: Methotrexate; MMF: Mycophemolate Mofetil; BM: Bone Marrow; PBSC: Peripheral Blood Stem Cells; CB: Cord Blood; cGvHD: Chronic Graft-Versus-Host Disease.*The cumulative incidence is calculated at 28 years.
Table 5: Analysis of factors predicting for SM.
Thirty-three patients (44.6%) out of 74 died at a median of 19 months (range, 1 to 212 months) after diagnosis of SM. Of them, 26 (78.8%) died because progression of SM and 7 (21.2%) died from other causes. Forty-one (55.4%) patients are living at a median of 79 months (range, 4 to 241 months) after SM diagnosis. Of them, 3 patients are now receiving chemotherapy and/or immunotherapy and thirty-eight are off therapy in complete remission of their malignancy. The ECOG performance score of 41 surviving patients is 0 for 35 (85%) patients, 1 for 3, 2 for 1, 3 for 1, and 4 for 1. The 35-yr Kaplan-Meyer OS and DFS for the entire cohort of patients with SM were 49.4% (95% CI, 32.8-63.2%) and 39.1% (95% CI, 27.9-55.7%) after a median time of survival of 31 years and 26.5 years, respectively (Figure 1B).
Incidence of Malignancy in Donors
Data on the malignancy occurrence were captured for family donors who were HLA genotypically identical or haploidentical with the patient. Unrelated donors (n=190) were excluded from the analysis because of the inability to obtain long-term follow-up information through the donor banks. The family donor population included 761 individuals (414 males, 54%) with a median age of 32 years (range, 0 to 70) at time of stem cell donation. Of them, 568 (74.7%) donors donated bone marrow stem cells (BM), 192 (25.2%) Peripheral Blood Stem Cells (PBSC) and 1 (0.1%) cord blood stem cells. At time of data-censoring date, 749 (98.4%) donors were living after a median follow-up of 18 years (range, 1 to 43 years). The follow-up completeness index for the entire cohort of family donors was 75% at 35 years since stem cell donation. Thirteen donors (1.7%) died over time, 8 due to malignancy and 5 for any other cause.
Among family donors, we observed 13 cases (1.7%) of malignancy, 7 in males and 6 in females, between July 2007 and February 2016, at a median of 15.04 years (range, 3 to 30.04 years) after stem cell donation and at a median age of 55 years (range, 25 to 74 years). Of them, 12 have donated BM and 1 PBSC. Type of malignancy, therapy and outcome are described in Table 4. Carcinoma of the breast (n=3) was the most common type of malignancy.
The cumulative incidence of malignancy for the entire cohort of family donors amounts to 0.2% (95% CI, 0.02-1.1%) at 10 years, 2.2% (95% CI, 1.0-4.3%) at 20 years, 4.8% (95% CI, 2.5-8.2%) at 30 years, and 5.8% (95% CI, 3.0-9.8%) at 35 years after stem cell donation (Figure 1A). As compared to the cumulative incidence of SM in transplant recipients, the cumulative incidence of malignancy in family donors was statistically lower [5.8% vs 17.0% (P=0.001)]. Of note, the two cohorts of 74 recipients with SM and 13 donors with malignancy were well matched in terms of age, gender, history of smoking, alcohol consumption, and drug abuse.

Figure 1a: Cumulative incidence of secondary malignancies in transplant recipients (continuous line) and malignancies in stem cell donors (dotted line).
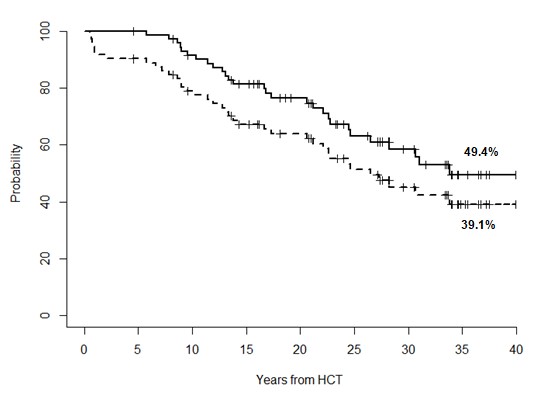
Figure 1b: Overall survival (continuous line) and disease-free-survival (dotted line) in transplant recipients with secondary malignancies.
In 4 cases the malignancy was diagnosed in the same recipient / donor couple. In 3 of the 4 cases the tumor was different (lung carcinoma in the patient and colon carcinoma in the donor, breast cancer in the recipient and prostate cancer in the donor, tongue cancer in the recipient and bladder cancer in the donor), while in one case recipient and donor had the same tumor i.e. non-Hodgkin lymphoma.
Eight (61.5%) of 13 donors died of tumor progression at a median of 12 months (range, 1 to 51 months) after onset of malignancy, and 5 are living after a median of 88 months (range, 61 to 181 months) after malignancy occurrence. All of them are free of malignancy with an ECOG score of 0. The 35-yr Kaplan-Meyer OS and DFS for the entire cohort of donors with malignancy was 33.7% (95% CI, 14.0-81.6%) after a median time of survival of 27.4 years.
Discussion
Patients treated with allogeneic HCT are at increased lifelong risk of developing SM. Table 6 describe recent large single-center and multi-center studies of SM in which the cumulative incidence of SM was calculated taking into consideration death without occurrence of malignancy as competing risk, as we did in the present study. The reported cumulative incidence of SM ranges from 1.0 to 8.7% at 10 years and from 3.3 to 13.9% at 20 years post-HCT. Very few studies published in the last two decades have described cumulative incidence data at 25 or more years after transplant. It is important to note that in general a plateau is not reached in long-term follow-up, and the cumulative incidence continues to increase over time. Male gender, cGvHD, older age at transplant, and irradiation were the main determinants for risk of SM. The most elevated excess absolute risk of developing SM was observed for tumors of the oral cavity and for melanoma of the skin. In our study we diagnosed SMs in 74 out of 951 patients (7.8%). The male-to-female distribution shows a slight male prevalence (40 males and 34 females).
Study
Study design
InstitutionSample
sizeConditioning
regimenMedian FU
months
(range)Number
of SMsCI of SM
at 10 years at 20 yearsMagnitude
of risk
SIRRisk factors
Michelis (22)
2007Single Centre
Ontario
2415
MAC 2035
RIC 380
127
(3-421)
151
6.3%
13.5%
2.07
Older age at HCT
Martelin (23)
2019Single Centre
Helsinki
1179
MAC 951
RIC 228
125
(28-256)
66
8.7%
13.9%
8.9%^
10.6%*
cGvHD, CML
Gallagher (6)
2007Single Centre
Vancouver
926
MAC 907
RIC 19
20
(0-230)
28
3.1%
-
1.85
Age > 40 years
Female donorRingden (9)
2104Multicentric
CIBMTR
4269
RIC
72
(1-188)
-
3.35%
-
0.99
Older age at HCT
Rizzo (8)
2009Multicentric
CIBMTR
28874
MAC
RIC
-
189
1.0%
3.3%
2.1
TBI, LFI, cGvHD
Atsuta (17)
2014Multicentric
JHSHCT
17545
MAC 13195
RIC 4141
-
269
1.7%
2.9%
1.8
cGvHD
HCT: Hemopoietic Cell Transplantation; FU: Follow-up; SMs: Secondary Malignancies; CI: Cumulative Incidence; SIR: Standardized Incidence Ratios (observed/expected ratios); CIBMTR: Center for International Blood and Marrow Research; RIC: Reduced Intensity Conditioning; MAC: Myeloablative Conditioning; cGvHD: Chronic Graft-Versus-Host Disease; CML: Chronic Myeloid Leukemia; TBI: Total Body Irradiation; LFI: Limited Field Irradiation; JSHCT: Japan Society for Hematopoietic Cell Transplantation; ^ melanoma; * oropharingeal cancer
Table 6: Recent large studies of second malignancy after allogeneic HCT.
The cumulative incidence of SM at 10 and 20 years after HCT in our study was similar to that observed in in other reported largest studies using the same statistical method for cumulative incidence, while treating death before SM occurrence as a competing risk. However, our data are important because the median follow-up of our survivors was long at 20 years, and this allowed us to estimate the cumulative incidence of SM at 30 and 35 years since transplantation. What appears relevant in our study is that the cumulative incidence of SM rises considerably over time, going from 5.6% at 20 years after transplantation until reaching a rate of 11.9% at 30 years and even 17% at 35 years without evidence of a plateau. This figure is very close to reality as the vast majority of our patients were updated in the last 3 months before the closure of the study. Moreover, of note in our study, we found that patients developed SM at younger age (median 47 years) if compared to that of the general population (over 60 years) and that of family donors (55 years) and after a median time from HCT to SM of 16.09 years.
In most of the previously published studies, the incidence of SM has been compared with cancer incidence observed in the general population by using standard incidence ratios [6,8-9,17,22-23]. Overall, transplant recipients developed an invasive solid cancer at twice the rate expected based on population incidence rates, as depicted in Table 6. The risk reached 3-fold among patients followed for 15 years or more after transplantation. Since data comparing the incidence of SM between allografted patients and the general population are widely available in most of the many scientific papers published and being able to have follow-up data of our family donors, we chose to compare the cumulative incidence of SM in HCT recipients with the cumulative incidence of malignancy in family donors. This choice allowed us to abrogate the factor related to the familial genetic predisposition which is a well-known risk factor for many cancers. The two groups were matched for age and gender. Even with the limitations of a slightly different median follow-up between transplant recipients and family donors (20 vs 18 years), we found that transplant recipients developed malignancies at triple the rate of malignancies observed in family donors (35-yr cumulative incidence 17.0% vs 5.8%) (P=0.001). Of relevance in our study, transplant recipients develop cancer at a much younger age than family donors (47 years vs 55 years), thus confirming the impact of the transplant and its related factors (chemotherapy and in particular alkylating agents, radiotherapy, GvHD, prolonged immunosuppression) on tumor development.
Among transplant recipients, squamous cell carcinoma of the oral cavity was the most common type of SM, whereas in the cohort of family donors carcinoma of the breast was predominant as in the general population. In a recent study, we have demonstrated that a diagnosis of hematological nonmalignant disease and duration of oral cGvHD for more than 15 months were factors significantly associated with higher incidence of squamous cell carcinoma of the oral cavity [18]. A particular mention must be reserved to the finding of an ever-increasing incidence of secondary malignancies in patients affected by thalassemia major. The data found in the present study (17 cases in 137 patients for a cumulative incidence of 21.7%), confirms and extends what we have previously reported in two other studies [19,20]. The incidence of malignancies in the cohort of family donors (13 cases in 761 donors, 1.7%) was apparently similar to that described by Pulsipher et al (26 cases in 2408 PB donors, 1.1%) [21].
Several risk factors have been identified that may contribute to the development of SM after HCT. Younger age at transplantation, male gender, use of total body irradiation in conditioning regimen, cGVHD, and immunodeficiency from incomplete recovery after transplant have been implicated as risk factors in some studies, while a strong association between these factors and second cancers has not been observed in others [22-25]. In our study the univariate analysis based on different and multiple risk factors estimated that cGvHD and its duration for more than 24 months were associated to a higher incidence of SM. However, the multivariate analysis showed that only cGvHD was an independent risk factor for development of SM with a hazard ratio of 2.85. The independent risk of cGvHD, as shown in many other series, highlights a stringent need to prevent this complication or treat it with novel therapies that don’t result, if possible, in prolonged immunosuppression. In any case, tumor screening every 6 months may be considered for patients at high risk for developing SM, such as patients with prolonged previous cGVHD.
Standard therapeutic strategies to treat SM after HCT are lacking because there are no large studies in this regard. With respect to outcome, some series indicate that the 5-year OS rates after diagnosis of SM varied by cancer site, with 88%-100% for thyroid, testis and melanoma, approximately 50% for breast, mouth, soft tissue and female reproductive organs, and 20% or less for bone, lung, lower gastrointestinal tract, and central nervous system [26,27]. These rates were similar to those of de novo cancers, except that rates were lower for female reproductive organs, bone, colorectum, and central nervous system. Our study shows similar results considering that, the 35-yr Kaplan-Meyer OS and DFS for the entire cohort of patients with SM were 49.4% and 39.1% after a median time of survival of 31 years and 26.5 years, respectively. A comprehensive study of a large number of patients with SM will help to determine the nature of these tumors and their outcomes compared with de novo tumors. Until then, patients with SM developing after HCT should be treated with the best available therapy for that tumor, unless there is compelling evidence that they will not be able to tolerate that therapy. Considering that the prognosis for patients with SM still remains poor despite therapeutic advances in the oncologic setting, early diagnosis and treatment remains to be the key to improving survival of patients [28-31]. For this purpose, an international working group was established through the Center for International Blood and Marrow Transplant Research Late Effects and Quality of Life Working Committee and the European Group for Blood and Marrow Transplantation Complications and Quality of Life Working Party with the goal to facilitate implementation of cancer screening appropriate to HCT recipients. As a clinical output of this working group’s effort, consensus-based recommendations applicable for screening and prevention of individual secondary solid cancers among HCT recipients were published in 2015 [30].
Important strengths of our study include its large size from a single center, the long median duration (20 years) and completeness (99% at 35 years) of follow-up for transplant recipients and a statistical analysis that included many risk factors potentially involved in the development of SM. Similar follow-up is difficult, if not impossible, to achieve through large multicenter allogeneic transplant registries. The present study is not conclusive, but extends our understanding of SM risk after HCT. However, the study has several limitations, mainly because of its retrospective design. A further weakness of the study is the lack of detailed information on family history of cancer and on the exposure of all recipients and all donors to well-known risk factors for malignancies such as smoking, alcohol consumption, drug abuse, use of chemicals and health-related lifestyle. The follow-up completeness index for the entire cohort of living recipients is different in transplant recipients (99%) than in family donors (75%). Moreover, other information is lacking on the specific treatments (chemotherapy, immunotherapy, radiotherapy) adopted for each patient affected by MS.
In conclusion, HCT offers curative therapy for many patients with otherwise incurable disease. The incidence of post-transplant SM appears high, although reliable estimates of the overall risk will require longer follow-up. Despite the lack of randomized studies, the benefit of HCT, as compared with conventional therapy alone, in certain clinical situations outweighs the risk of late SM. It is imperative to follow HCT recipients closely and screen them for the development of SMs to decrease the morbidity and mortality associated with this complication.
Author Statements
Acknowledgements
The authors acknowledge the contribution of the outstanding team of the medical, nursing, technician staff at the UOSD Terapia Intensiva Ematologica and the UOSD Istituto dei Tessuti e Biobanche. Special thanks also are reserved to all the patients with their families.
Author’s Contribution
S.S., P.D.B. contributed patients, designed the study, analyzed the data, and wrote the manuscript; A.N., P.O., D.V., R.S. contributed to data acquisition and analysis; S.A. performed statistical study and contributed to the interpretation of the results.
Conflict of Interest Disclosure
All authors declare no competing financial interest.
References
- Baker KS, DeFor TE, Burns LJ, Ramsay NKC, Neglia JP, Robison LL. New malignancies after blood or marrow stem cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003; 21: 1352-8.
- Majhail NS, Brazauskas R, Rizzo JD, Sobecks RM, Wang Z, Horowitz MM et al. Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan–cyclophosphamide conditioning. Blood. 2011; 117: 316-22.
- Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socíe G, Travis LB, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997; 336: 897-904.
- Bhatia S, Louie AD, Bhatia R, O’Donnell MR, Fung H, Kashyap A, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001; 19: 464-71.
- Shimada K, Yokozawa T, Atsuta Y, Kohno A, Maruyama F, Yano K, et al. Solid tumors after hematopoietic stem cell transplantation in Japan: incidence, risk factors and prognosis. Bone Marrow Transplant. 2005; 36: 115-21.
- Gallagher G, Forrest DL. Second solid cancers after allogeneic hematopoietic stem cell transplantation. Cancer. 2007; 109: 84-92.
- Socié G, Henry-Amar M, Bacigalupo A, Hows J, Tichelli A, Ljungman P, et al. Malignant tumors occurring after treatment of aplastic anemia. European bone marrow transplantation-severe aplastic anaemia working party. N Engl J Med. 1993; 329: 1152-7.
- Rizzo JD, Curtis RE, Socié G, Sobocinski KA, Gilbert E, Landgren O, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009; 113: 1175-83.
- Ringdén O, Brazauskas R, Wang Z, Ahmed I, Atsuta Y, Buchbinder D, et al. Second solid cancer after hematopoietic cell transplantation using reduced intensity conditioning. Biol Blood Marrow Transplant. 2014; 20: 1777-84.
- Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009; 15: 1628-33.
- Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus- host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974; 18: 295-304.
- Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003; 9: 215-33.
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999; 94: 496-509.
- Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988; 16: 1141-54.
- Therneau TM, Grambsch PM, Fleming TR. Martingale-based residuals for survival models. Biometrika. 1990; 77: 147-60.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958; 53: 457-81.
- Atsuta Y, Suzuki R, Yamashita T, Fukuda T, Miyamura K, Taniguchi S, et al. Continuing increased risk of oral/esophageal cancer after allogeneic hematopoietic stem cell transplantation in adults in association with chronic graft-versus-host disease. Ann Oncol. 2014; 25: 435-41.
- Santarone S, Natale A, Angelini S, Papalinetti G, Vaddinelli D, Di Bartolomeo A, et al. Secondary oral cancer following hematopoietic cell transplantation. Bone Marrow Transplant. 2021; 56: 1038-46.
- Santarone S, Pepe A, Meloni A, Natale A, Pistoia L, Olioso P, et al. Secondary solid cancer following hematopoietic cell transplantation in patients with thalassemia major. Bone Marrow Transplant. 2018; 53: 39-43.
- Santarone S, Angelini S, Natale A, Vaddinelli D, Spadano R, Casciani P, et al. Survival and late effects of hematopoietic cell transplantation in patients with thalassemia major. Bone Marrow Transplant. 2022; 57: 1689-97.
- Pulsipher MA, Chitphakdithai P, Miller JP, Logan BR, King RJ, Rizzo JD, et al. Adverse events among 2408 unrelated donors of peripheral blood stem cells: results of a prospective trial from the National Marrow Donor Program. Blood. 2009; 113: 3604-11.
- Michelis FV, Kotchetkov R, Grunwald RM, Azeem A, Atenafu EG, Lipton JH, et al. Long-term incidence of secondary malignancies after allogeneic hematopoietic cell transplantation: a single-center experience. Biol Blood Marrow Transplant. 2017; 23: 945-51.
- Martelin E, Volin L, Itälä-Remes M, Niittyvuopio R, Lindström V, Heiskanen J, et al. Incidence and risk factors of secondary cancers after allogeneic stem cell transplantation: analysis of a single centre cohort with a long follow-up. Bone Marrow Transplant. 2019; 54: 334-7.
- Yokota A, Ozawa S, Masanori T, Akiyama H, Ohshima K, Kanda Y, et al. Secondary solid tumors after allogeneic SCT in Japan. Bone Marrow Transplant. 2012; 47: 95-100.
- Morton LM, Saber W, Baker KS, Barrett AJ, Bhatia S, Engels EA, et al. National Institutes of Health hematopoietic cell transplantation late effects initiative: The Subsequent Neoplasms Working Group Report the Subsequent Neoplasms Working Group report]. Biol Blood Marrow Transplant. 2017; 23: 367-78.
- Ehrhardt MJ, Brazauskas R, HE W, Rizzo JD, Shaw BE. Survival of patients who develop solid tumors following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2016; 51: 83-8.
- Inamoto Y, Matsuda T, Tabuchi K, Kurosawa S, Nakasone H, Nishimori H, et al. Outcomes of patients who developed subsequent solid cancer after hematopoietic cell transplantation. Blood Adv. 2018; 2: 1901-13.
- Bhatia S, Francisco L, Carter A, Sun CL, Baker KS, Gurney JG, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the bone marrow transplant Survivor Study. Blood. 2007; 110: 3784-92.
- Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012; 18: 348-71.
- Inamoto Y, Shah NN, Savani BN, Shaw BE, Abraham AA, Ahmed IA, et al. Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Transplant. 2015; 50: 1013-23.
- Tichelli A, Beohou E, Labopin M, Socié G, Rovò A, Badoglio M, et al. Evaluation of second solid cancers after hematopoietic stem cell transplantation in European patients. JAMA Oncol. 2019; 5: 229-35.