
Research Article
Austin J Environ Toxico. 2024; 10(1): 1048.
Antibacterial Activity of Processed and Unprocessed Honey Samples Against the Clinical Bacterial Pathogens from Kanhangad, Kasaragod District, KL, India
Usharani K1,2*; Ummul Mainoor1; Fathimath Thahira1; Ayshath Rahida1
¹Department of Environmental Science, School of Energy, Environment and Earth Sciences, Central University of Kerala, India
²Department of Civil and Environmental Engineering, Universidade Estadual Paulista Julio De Mesquita Filho, Bauru, Brazil
*Corresponding author: Usharani K Research Fellow, Department of Civil and Environmental Engineering, Universidade Estadual Paulista Julio De Mesquita Filho, Bauru, Brazil. Email: usharaniks2003@yahoo.com
Received: January 12, 2024 Accepted: February 16, 2024 Published: February 23, 2024
Abstract
The study intended to either kill or inhibit the growth of these pathogens in the milk by adding antibiotic substances from natural sources without denaturing the quality of the food material. The study aimed to determine the broad spectrum of antimicrobial activity of honey from two different sources natural (unprocessed honey) and commercial (processed honey). The inhibitory action of extracts of honey was evaluated against six bacterial pathogenic strains, Escherichia coli, Enterobacter cloacae Pseudomonas aeruginosa, Proteus mirabilis, Enterococcus faecium, and Staphylococcus aureus by agar well diffusion method. The minimum inhibitory concentration of bactericidal activity was estimated and the presence of bioactive compounds by spectral analysis using a UV-visible spectrophotometer. The results were obtained by measuring the zones around the wells after the diminution of the well size. It was found to be positive results on cultures using honey samples. The growth of the undiluted culture was less inhibited by different honey samples and the diluted was highly inhibited by all honey samples, and the number of bacterial colonies was high in the undiluted culture whereas less in the diluted culture. The extract of honey showed better antibacterial activities contrary to pathogenic bacteria. Hence, it revealed that as the dilution rate increased, the antibiotic sensitivity also increased. The honey samples capable of antibiotic activity against clinical pathogens have the potential to be used as an effective tool for inhibiting the growth of pathogenic microorganisms and potentially used as a promising application in antibacterial agent against pathogens of raw milk.
Keywords: Honey; Agar well diffusion assay; Minimum Bactericidal Concentration
Introduction
According to the World Health Organization (WHO) measurements, up to 80% of the population in some established countries have used natural products in their primary health care [22,38]. Furthermore, 80% of people are influenced by these types of treatment in Asian countries including India [22]. The researchers have found that natural materials are normally more acceptable to users, and if these substitute methods are in effect, this may decrease the support for other synthetic substances [32]. Also, the study of such natural compounds may lead to the finding of a bioactive component that could prevent some ecological threats or effects on a disease process in humans [17]. The growth in the resistance of pathogenic bacteria to antibiotics is too a progressively vital factor behind the growing interest in the use of these natural compounds. The herbs, plant extracts, essential oils, and honey are the supreme collective sources for these bioactive compounds [17], and these products are effective against a range of bacterial infections and inflammatory cases [1,21].
Honey is the natural sweet substance produced by honeybees from the nectar of blossoms or from secretions of living parts of plants or excretion of plant-sucking insects on the active parts of plants, which honeybees gather, convert, and pool with specific substances of their own, store and leave in the honeycomb to mature and ripen. The honey is ranked by color, with the clear, golden amber honey often at a higher trade price than darker varieties. The flavor of honey will vary based on the types of flower from which the nectar was yielded. The presence of its nutritive, therapeutic, and dietetic quality in honey, is used in the food industry, medicine, and many other domains. From the time when prehistoric periods, it was discovered that honey is a medicine and is well known for its antibacterial activity, which was first reported in 1892 [31,20]. And also it has been used for the treatment and prevention of wound infections. With the advent of antibiotics, the medical application of honey was dropped in current Western medicine, though it is still used in traditional ways. For all antibiotic classes, including the major last-resort drugs, resistance is increasing worldwide and even more alarming, very few new antibiotics are being developed. The potential activity of honey against antibiotic-resistant bacteria resulted in renewed interest in its application and has been approved for medicinal application. The inadequate knowledge of the antibacterial compounds and the changeability of antibacterial activity are chief hindrances to the applicability of honey in medicine. In current years, the information on the antibacterial compounds in honey has expanded.
Nowadays, a lot of people group honey for its antibacterial and anti-inflammatory properties. The complete practitioners think through its unique nature's greatest versatile remedies [27]. The high sugar concentration, hydrogen peroxide, and the low pH are renowned antibacterial influences in honey, and further lately, methylglyoxal and the antimicrobial peptide bee defensin-1 and also phytochemical compounds, such as aromatic acids and phenols [2,6,27] Hegazi et al. 2017) were recognized as vital antibacterial compounds in honey. The antibacterial activity of honey is extremely composite due to the association of manifold complexes and due to the great difference in the concentrations of these composites among honey [28,29]. The information that honey has antibacterial properties has been documented for more than a century since it therapies for infections [34]. The honey’s resistance has not ever been stated nor has any toxicity effects, low cost of maintenance and local availability confer valuable advantages to using honey as a substitute antimicrobial therapy [40]. There is much information on the antimicrobial activity of honey against a wide range of bacterial and fungal species [7,14]. The antibacterial activity of honey performance has been stated first by van Ketel (1892), followed by Dustmann (1919). The following statement was by Sacket (1919), he also described that the antibacterial potency was augmented by limited dilution of honey, a comment that was hard to describe. The additional serious study did not begin until the work of Dold et al (1937). They make known the term 'inhibin' for the antibacterial activity of honey, a term which has been extensively used subsequently in the literature on honey. Later then there have been several accounts, some have been of simple testing that has revealed honey to have antibacterial activity, and these have often been done without credit of the prior finding of this by others. Furthermost, the study involved the activity spectrum of honey (i.e. defining which species of microorganism are sensitive to the action of honey) or comparison of different types of honey for the effectiveness of their action against one or additional species of bacteria. The practice of milk is quite common in our day-to-day activities. While using raw milk or even after boiling some of the pathogens remain in it and lead to some common infection along with other immune weakness either directly or indirectly. Therefore, the study intended to either kill or inhibit the growth of these pathogens in the milk by adding antibiotic substances from natural sources without denouncing the quality of the food material. The study aimed to determine the broad spectrum of antimicrobial activity of honey from two different sources natural (unprocessed honey) and commercial (processed honey). The screening and selection of pathogens are based on the literature survey and report from the Milma dairy industry (Kanhangad, KL, India). Hence, honey was selected as a natural antibiotic compound with the following properties; its medicinal properties, immunomodulatory properties, presence of glucose oxidase (Peroxide effect), and its availability.
Materials and Methods
Sample collection
The honey samples of three unprocessed and three processed were obtained from different sources and coded as follows respectively, Natural (N1), Aralam (N2), Jamun (N3), Lion (A1) Begood (A2), Dabur (A3), from Kasaragod District. All samples were kept in sterile screw cap tubes and stored at dark at room temperature.
Pathogen Selection and Collection
The pathogenic bacteria used in this study were Escherichia coli (a), Enterobacter cloacae (b), obtained from Kanhangad Diagnostic Centre (KDC) Lab (Kanhangad). These were subcultured on Nutrient agar and incubated aerobically at 37°C. Organisms were maintained in the laboratory on nutrient broth at 37°C for 24 h and then kept at 4°C before further experiments.
Serial Dilution and Estimation of Microbial Concentration
The serial dilution and pour plate method was used for the study using samples from pure cultures of bacteria. A liquid bacterial culture was inoculated and spread onto the surface of the agar plate. To ensure uniformity of sample distribution on the plate spreading in this instance, it should be done with a glass rod and not by the heavy streak method. The plate was incubated to allow bacterial growth and colonies were counted. Since every cell in the population will proliferate to a visible colony, the colonies on the plate characterize the number of cells that exist in the sample taken from the population. A 100μl sample was taken from the flask and placed in 900μl of water in tube A; the contents of tube A would represent a 10-fold dilution of the original sample. That is, the cell number per μl would be 1/10th of the original concentration. If 100μl was taken from tube A and placed into 900μl of water in tube B, that is another 10-fold dilution and represents a total concentration decrease of 1/100 from the original. If 100μl from tube B is placed in 900μl water in tube C, that is another 10- fold dilution now represents a 1/1000 concentration decrease of the original. Such dilution can be prepared up to 10 times dilution. Spread 100 μl samples from each tube into a culture plate and incubate for 24 hours to count the number of colonies.
Agar Plate Assay
In agar plate assay, for determining the concentration of bacteria used for the antibiotic activity of honey samples, therefore different dilutions were performed and serial dilution was done. For that, 100μl of broth was added into 900μl of deionized water and serially diluted. The 100μl of samples were transferred into sterile Petri dishes. The 20ml of nutrient agar medium was added. Also, the concentrations of pathogens at different dilutions were examined.
Broth Assay
Similarly for broth assay, to determine the bacterial concentration, 100μl of honey was added to 900μl of broth and serially diluted. The 100μl of each dilution was poured into a sterile Petri plate. Add 20ml of nutrient agar medium was added and left into laminar airflow for 24 hours. Then the concentration of pathogens at different dilutions was calculated.
Antibiotic Activity by well Diffusion Assay
The nutrient agar medium was prepared and poured into a sterile petri dish and left for solidification. Wells of 1cm diameter were made smoothly using a sterile test tube with 1cm diameter. Add 100μl of pathogenic bacterial culture as inoculum to nutrient agar medium into each petri plate and mix by using the spread plate method. After that, 100 μl of collected honey samples were poured into the wells individually and kept at 37°C for 24h. The results were expressed by measuring the zones around the wells after the diminution of the well size. The experiment was done in duplicate and the mean with standard deviation was calculated. Conferring to CLSI guidelines, these methods are often active in microbiological laboratories. For example, the agar diffusion assay procedure comprises smearing a petite volume of honey or a honey solution to the center of a well bored into a nutrient agar plate that has previously been inoculated with a bacterial culture. The honey disperses out into the agar from its application position while the plate is incubating. The zone of inhibition (ZOI), a clear zone adjacent to the honey application location, is a measure of the honey's efficacy [22].
Evaluation of Antibacterial Activity for the Presence of Hydrogen Peroxide or Proteinaceous Composites
The MIC values of the honey types treated with bovine catalase or proteinase K were evaluated and related to those of unprocessed honey. Concisely, 50% v/v honey in Muller- Hinton broth containing 100 μg/ml proteinase K or 600 U/ml bovine catalase was incubated for 16 h at 37°C, and then it was two-fold diluted and tested. The raised MIC values of the processed honey compared to the unprocessed honey showed the presence of hydrogen peroxide or proteinaceous compounds which supported the antibacterial activity of the verified honey types [24].
Influence of Individual Components on the Antibacterial Activity of Honey by Spectral Analysis
The diluted honey samples were analyzed for bioactive compounds by spectral analysis using a UV-visible spectrophotometer (UV-2600, SHIMADZU). The diluted samples were centrifuged at 5000 rpm for 4 hours and the catalyzed samples were subjected to spectral analysis.
Results and Discussion
The antimicrobial activity and Inhibition Zone Diameter of (IZD) of two different sources of Natural (unprocessed honey) and Commercial (processed honey) were determined for Escherichia coli and Enterobacter cloacae. The pathogens used in this study included Escherichia coli, Enterobacter cloacae Pseudomonas aeruginosa, Proteus mirabilis, Enterococcus faecium, and Staphylococcus aureus. Both natural and commercial were effective against Escherichia coli and Enterobacter cloacae. The effect on Escherichia coli is somewhat more than Enterobacter cloacae. In this study, six honey samples were tested for their antimicrobial activity on Escherichia coli and Enterobacter cloacae. The present study showed varying degrees of growth inhibition activity of Natural and Commercial honey samples against the tested organisms. These might be due to the osmotic effect and the sensitivity of these organisms to hydrogen peroxide which are unsuitable for bacterial growth and represented as an inhibition factor in honey [19,30].
Honey has several well-known characteristics that are generally accepted as contributing to total antimicrobial activity. These include low pH, an osmotic effect, hydrogen peroxide production, and phytochemical factors [9]. The antimicrobial activity of many honeys can be attributed predominantly to hydrogen peroxide activity [13], evidenced by a decline in antimicrobial activity after treatment with the enzyme catalase. The medicinal effects of honey date back to the days of Aristotle (384-322 BC) for the treatment of sore eyes and wound infections [16,36]. The antimicrobial characteristics of honey have been established for a long time especially for wound healing [8,36]. Its activity may be due to its complex composition and its ability to generate hydrogen peroxide by the bee-derived enzyme glucose oxidase [4,12,15,33,36]. Regarding the number of strains sensitive to the action of honey, a relationship, and interactions between the origin of honey and its antibacterial activity was observed. The natural (N1, N2, N3) samples present a high antibacterial activity against all bacterial strains. The commercial (A1, A2, A3) also have high antibacterial activities. These findings confirm the ability of various types of honey to inhibit strains of pathogenic bacteria whether susceptible or resistant to standard antibiotics. The antibacterial activity of honey was different according to the area of origin. To maximize the therapeutic effects of honey, a careful selection of those with high levels of antibacterial activity must be made. This is possible by identifying the factors that increase the antibacterial effect. In the meantime, the isolation of honey’s active constituents may underlie the synthesis of new drugs with antibacterial effects.
Serial Dilution Method
The concentrations of the bacteria present in different dilutions were determined using serial dilution. Table .1, shows the concentration of pathogens Escherichia coli, Enterobacter cloacae Pseudomonas aeruginosa, Proteus mirabilis, Enterococcus faecium, and Staphylococcus aureus present in different dilutions. The table reveals that the concentration of pathogens decreases when going for dilution, which means the number of bacterial colonies was more at dilution 10-1 with 356x10 (CFU/μl) in Escherichia coli and 252x10(CFU/μl) in Enterobacter cloacae. Similarly, less number of bacterial colonies are present at 10-5 dilution with 40x105(CFU/μl) in E.coli and 32x105(CFU/μl) in Enterobacter cloacae.
Sample
Dilution
Dilution factor
Number of colonies
Average colony forming unit (CFU/μl)
Escherichia coli
1
10-1
101
89x4
356x10
2
10-2
102
45x4
180x10²
3
10-3
103
32x4
128x10³
4
10-4
104
20x4
80x104
5
10-5
105
10x4
40x105
Enterobacter cloacae
1
10-1
101
63x4
252x10
2
10-2
102
47x4
188x10²
3
10-3
103
30x4
120x10³
4
10-4
104
15x4
60x104
5
10-5
105
8x4
32x105
Pseudomonas aeruginosa
1
10-1
101
156x4
624x10
2
10-2
102
98x4
392x10²
3
10-3
103
73x4
292x10³
4
10-4
104
39x4
156x104
5
10-5
105
10x4
40x105
Proteus mirabilis
1
10-1
101
55x4
220x10
2
10-2
102
44x4
176x10²
3
10-3
103
40x4
160x10³
4
10-4
104
35x4
140x104
5
10-5
105
18x4
72x105
Enterococcus faecium
1
10-1
101
53x4
212x10
2
10-2
102
36x4
144x10²
3
10-3
103
24x4
96x10³
4
10-4
104
17x4
68x104
5
10-5
105
8x4
32x105
Staphylococcus aureus
1
10-1
101
88x4
352x10
2
10-2
102
70x4
280x10²
3
10-3
103
27x4
108x10³
4
10-4
104
19x4
75x104
5
10-5
105
10x4
40x105
Table 1: Concentration of pathogens at different dilution.
Antibiotic Activity using Agar Plate Assay by well Diffusion Method
In the good diffusion method, all honey samples from different sources showed inhibitory activity against all the target pathogenic bacteria; the growth inhibitory zone varies between 1.2 and 3.6 cm (Table 2). The antimicrobial activity of honey was studied both in Undiluted and Diluted cultures of clinical pathogens. The diameter of inhibition zones was studied and the results were illustrated in Tables (1 and 2). The lesser the dilution, the greater the decrease in antimicrobial activity against Escherichia coli and Enterobacter cloacae strains. Among the bacteria evaluated, Escherichia coli were easily inhibited by all tested honey samples. Growth of Escherichia coli was easily inhibited by Be Good honey (A2), Lion honey (A1), Dabur honey (A3), Jamun honey (N3), Aralam honey (N2), Natural honey, Kasaragod (N1) with the inhibitory zone of 3.6, 3.6, 3.5, 3.2, 3.2, 2.7 cm, respectively (Table.2). From the Table.2 growth of Enterobacter cloacae was moderately inhibited by all honey samples with inhibitory zone between the ranges of 1.2 to 2.5cm. The honey samples unprocessed honey (N1, N2, and N3) and processed (A1, A2, and A3) showed antibiotic sensitiv ity of 1.2, 1.4, 2.1, 2.2, and 2.5cm against Enterobacter cloacae of 260x103 (CFU/μl) numbers of cells. From Table 2, it can be interpreted that Be Good Honey (A2) showed the highest inhibitory activity against tested pathogenic bacteria (3.6cm in E. coli and 2.5cm in Enterobacter cloacae). The above table revealed different antibiotic sensitivity in E.coli and Enterobacter cloacae broth cultures with different honey samples. Here, Natural honey (N1) Kasaragod showed less antibiotic activity on these bacterial cultures with antibiotic sensitivity of 2.7cm and 1.2cm in E.coli and Enterobacter cloacae because of the absence of preservatives in Natural honey (N1). Hence, Natural honey (N1) Kasaragod was observed to be the purest one. From tables, revealed that, In the case of Escherichia coli, the Antibiotic activity of Natural(N1) honey, Kasaragod showed 2.7cm as a zone of inhibition under Undiluted culture of 363x10³(CFU/μl) and 1.3 cm zone of inhibition under Diluted culture of 356x10(CFU/μl) strains. They show a 1.4 cm variation between them. The honey samples of Aralam (N2) and Jamun (N3) honey showed same antibiotic sensitivity of 3.2 cm in undiluted culture and 3.8 and 3.3 cm of antibiotic sensitivity in diluted culture. There were differences of 0.6 cm and 0.1 cm. Antibiotic activity of Lion(A1) honey and Be good(A2) showed the same zone of inhibition against undiluted culture, which was 3.6 cm, and under a diluted culture of E.coli, Lion and Be good honey showed antibiotic sensitivity of 3.8 and 3.9 cm, which gives differences of 0.2 and 0.3 cm. At last, the Dabur (A3) honey showed antibiotic sensitivity of 3.5 cm in undiluted culture and 3.6 cm in diluted culture with 0.1cm. Thus, the antibiotic activity of different honey samples against the diluted culture of Escherichia coli was higher than that of the antibiotic activity of honey against undiluted culture. The antibiotic and antibacterial activity of honey against clinical pathogens by well diffusion method is shown in Table 2. In the good diffusion method, for undiluted culture six honey samples from different sources showed inhibitory activity against target pathogenic bacteria. In the good diffusion method, all honey samples from different sources showed inhibitory activity against all the target pathogenic bacteria; the growth inhibitory zone varies between
Sample
Average colony forming unit (CFU/100μl)
Diameter of zone (Z1,cm)
Diameter of well (W1,cm)
Antibiotic Sensitivity (Z1-W1,cm)
Escherichia coli
N1
363x10³
3.7
1
2.7
N2
363x10³
4.2
1
3.2
N3
363x10³
4.2
1
3.2
A1
363x10³
4.6
1
3.6
A2
363x10³
4.6
1
3.6
A3
363x10³
4.5
1
3.5
Enterobacter cloacae
N1
260x10³
2.2
1
1.2
N2
260x10³
2.4
1
1.4
N3
260x10³
3.1
1
2.1
A1
260x10³
3.2
1
2.2
A2
260x10³
3.5
1
2.5
A3
260x10³
3.5
1
2.5
Pseudomonas aeruginosa
N1
631x10³
2.2
1
1.2
N2
631x10³
3.4
1
2.4
N3
631x10³
3.4
1
2.4
A1
631x10³
3.5
1
2.5
A2
631x10³
4.0
1
3.0
A3
631x10³
4.2
1
3.2
Proteus mirabilis
N1
228x10³
2.5
1
1.5
N2
228x10³
3.4
1
2.4
N3
228x10³
3.6
1
2.6
A1
228x10³
3.8
1
2.8
A2
228x10³
3.8
1
2.8
A3
228x10³
4.0
1
3.0
Enterococcus faecium
N1
219x10³
2.2
1
1.2
N2
219x10³
2.4
1
1.4
N3
219x10³
2.6
1
1.6
A1
219x10³
3.4
1
2.4
A2
219x10³
3.4
1
2.4
A3
219x10³
3.5
1
2.5
Staphylococcus aureus
N1
359x10³
2.6
1
1.6
N2
359x10³
3.9
1
2.9
N3
359x10³
4.0
1
3.0
A1
359x10³
4.1
1
3.1
A2
359x10³
4.2
1
3.2
A3
359x10³
4.5
1
3.5
Unprocessed honey: N1: Natural honey, Kasaragod; N2: Aralam honey; N3: Jamun honey; Processed honey: A1: Lion honey; A2: Be good honey; A3: Dabur honey
Table 2: Antibiotic activity of different samples against clinical pathogens.
1.2cm and 3.5cm (Table 2). From Table 2, the Antibiotic activity of different honey samples of Undiluted and Diluted cultures of Escherichia coli and Enterobacter cloacae showed differences in antibiotic sensitivity values. In the case of undiluted cultures of Escherichia coli and Enterobacter cloacae, Escherichia coli is highly inhibited by all honey samples than Enterobacter cloacae. E. coli showed 2.7 to 3.5 cm of inhibition zone diameter whereas Enterobacter cloacae showed 1.2 to 2.5 cm of inhibition zone diameter. In Diluted cultures of Escherichia coli and Enterobacter cloacae, also E.coli showed higher inhibitory activity in all honey samples than Enterobacter cloacae. Here E.coli showed inhibition zone diameter of 1.3 to 3.6 cm whereas Enterobacter cloacae showed inhibition zone diameter of 1 to 1.5 cm.
Table. 2, revealed that , In case of Escherichia coli, the Antibiotic activity of Natural(N1) honey, Kasaragod showed 2.7cm as a zone of inhibition under Undiluted culture of 363x10³(CFU/μl) and 1.3 cm zone of inhibition under Diluted culture of 356x10(CFU/μl) strains. They show a 1.4 cm variation between them. The honey samples of Aralam (N2) and Jamun (N3) honey showed same antibiotic sensitivity of 3.2 cm in undiluted culture and 3.8 and 3.3 cm of antibiotic sensitivity in diluted culture. There were differences of 0.6 cm and 0.1 cm. Antibiotic activity of Lion(A1) honey and Be good(A2) showed the same zone of inhibition against undiluted culture, which was 3.6 cm, and under diluted culture of E.coli, Lion and Be good honey showed antibiotic sensitivity of 3.8 and 3.9 cm, which gives differences of 0.2 and 0.3 cm. At last, the Dabur (A3) honey showed antibiotic sensitivity of 3.5 cm in undiluted culture and of 3.6 cm in diluted culture with 0.1cm. Thus, the antibiotic activity of different honey samples against the diluted culture of Escherichia coli was higher than that of the antibiotic activity of honey against undiluted culture. Among the studied bacteria, Pseudomonas aeruginosa and Proteus mirabilis showed inhibitory activity by honey samples (Table 2). In both organisms Dabur (A3) honey showed the highest inhibitory activity. In the case of Pseudomonas aeruginosa, Natural Kasaragod honey (N1) showed inhibitory activity of 2.2 cm and Dabur (A3) showed activity of 4.2 cm. In the case of Proteus mirabilis Natural Kasaragod honey (N1) showed inhibitory activity of 1.5cm and Dabur (A3) showed activity of 3cm (Table 2). From the results, the inhibitory activity of Pseudomonas aeruginosa and Proteus mirabilis were observed. The Dabur (A3) honey showed inhibitory activity of 3.2 cm and 3cm respectively. From the table, it can be interpreted that Dabur (A3) honey showed the highest inhibitory activity against tested pathogenic bacteria., natural Kasaragod honey (N1) showed the least(1.2cm and 1.5cm) inhibitory activity, which means there is no added preservatives present in the Natural Kasaragod honey (N1) and it is hundred percent pure. Among the bacteria evaluated, Staphylococcus aureus was easily inhibited by all tested honey samples. Growth of Staphylococcus aureus was easily inhibited by Dabur honey (A3), Be good honey (A2), Lion honey (A1), Jamun honey (N3), Aralam honey (N2), Natural honey, Kasaragod (N1) with inhibitory zone of 3.5, 3.2, 3.1, 3, 2.9 and 1.6 cm, respectively as shown in Table.2. Table 2, represent the antibiotic activity of different honey samples such as N1, N2 and N3 (Natural-unprocessed honey) and A1, A2, and A3 (Commercial-processed honey) against Enterococcus faecium and Staphylococcus aureus. It was observed that Dabur honey (A3) showed the highest inhibitory activity against Enterococcus faecium and Staphylococcus aureus (3.3 and 2.5 cm), while Natural honey, Kasaragod was effective against Enterococcus faecium and Staphylococcus aureus (1.2 and 1.6cm) showed less activity. Thus the antibiotic activity of Be good (A2) honey and Lion (A1) honey against Escherichia coli was higher compared to other honey samples with an inhibitory zone of 3.6 cm in 363 x103 (CFU/μl) concentration of broth. From the figure.1, it revealed that growth of Escherichia coli, Enterobacter cloacae, Pseudomonas aeruginosa, Proteus mirabilis, Enterococcus faecium, and Staphylococcus aureus were moderately inhibited by all honey samples. From the figure, the Natural honey Kasaragod (N1) shows less activity, and Dabur honey (A3) and Be good honey (A2) show higher antibiotic activity against Enterobacter cloacae. Hence, Dabur honey (A3) and Be good honey (A2) were highly effective which was 2.5 cm. Among the figures (1; 2a-2f; 3a-3b), Escherichia coli was highly inhibited and showed high antibiotic sensitivity with different honey samples than Enterobacter cloacae. From the figures (1; 2a-2f; 3a-3b) it was observed that Natural honey (N1) Kasaragod (unprocessed honey) showed less antibiotic sensitivity than commercial honey samples (processed honey), which revealed that the processed honey samples contain additives or preservatives which inhibit the growth of cultures. Thus the unprocessed honey samples were more effective. The antibiotic sensitivity of different honey samples against Pseudomonas aeruginosa. From the figure, the commercial (Processed honey; A1, A2, A3) honey showed high antibiotic sensitivity tan Natural (Unprocessed honey; N1, N2, N3) honey against Pseudomonas aeruginosa. The Natural Kasaragod (N1) honey showed the least activity with an inhibitory zone of 1.2cm and Dabur (A3) honey showed the highest activity with an inhibitory zone of 3.2cm. The increase in the activity of different honey samples against Proteus mirabilis. From the figure, the Natural Kasaragod honey (N1) showed less (1.5cm) inhibitory activity and Dabur (A3) honey showed the highest activity with an inhibitory zone of 3cm.This means Dabur (A3) honey is very effective against Proteus mirabilis. Table .2 shows the inhibitory activity of all honey samples from different sources against target pathogenic bacteria. It shows an increase in antibiotic sensitivity with an increase in dilution from 10-1 to 10-5. The growth inhibitory zone varies from 1 to 4.5 cm. The strain Escherichia coli showed high sensitivity to all honey samples. The diameter of the inhibition zone observed for Escherichia coli is 4.5 cm at 10-5 dilution using N2: Aralam honey, the highest value among all the samples and studied strains. The results are very important from the clinical point of view, these bacterial strains (E.coli) play an important role as an epidemiological causative agent of diarrhea in children and calves. Thus, honey can inhibit the growth of Escherichia coli. Regarding the activity of honey against Enterobacter cloacae, the growth were moderately inhibited by all honey samples.
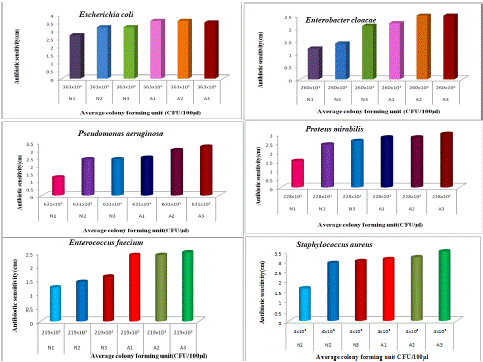
Figure 1: The antibiotic activity of unprocessed honey (Natural honey Kasaragod (N1), Aralam (N2), Jamun (A3) and processed honey Lion (A1), Be good (A2), Dabur (A3) honey samples against clinical pathogens.
From Table 2, Enterobacter cloacae showed less antibiotic sensitivity of 1cm at a concentration of 10-1 in Natural (N1) honey, Kasargod. Regarding the sensitivity to the other five honey samples, the diameters of the inhibition zones at different concentrations are quite low (1, 1.6, and 1.7 cm) for Enterobacter cloacae using Be good (A2) honey. The antibiotic sensitivity of honey samples N1, N2, N3 (natural honey) and A1, A2, A3 (commercial honey) against diluted cultures of Pseudomonas aeruginosa and Proteus mirabilis. In the case of both organisms as dilution increased number of organisms decreased and there was an increase in the antibiotic sensitivity of honey samples from 10-1 to 10-5 dilutions. The antibiotic sensitivity of natural Kasaragod (N1) and Aralam (N2) honey samples against clinical pathogens. The Pseudomonas aeruginosa showed the least inhibitory activity (1.4cm) in 10-1 dilution and the highest inhibitory activity (3.6cm) in 10-5 dilution. The Proteus mirabilis showed 1.3cm and 3cm for 10-1 and 10-5 dilutions respectively.
The antibiotic sensitivity of Jamun (N3) and Lion (A1) honey samples against clinical pathogens was recorded as mentioned in Figure 2c. As the dilution rate increased, the number of organisms was decreased and the antibiotic sensitivity was increased. Pseudomonas aeruginosa showed the least inhibitory activity (2cm) in 10-1 dilution and the highest inhibitory activity (3.4cm). And that of Proteus mirabilis is 2cm and 3cm for 10-1 and 10-5 dilutions respectively. The antibiotic sensitivity of Begood (A2) and Dabur (A3) honey samples against clinical pathogens. In both organisms, 10-1 dilution showed the least inhibitory activity and 10-5 dilution showed the highest inhibitory activity. In the case of Begood (A2) honey, Pseudomonas aeruginosa showed inhibitory activity of 1.5 and 2.6cm at 10-1 and 10-5 dilutions respectively. Proteus mirabilis showed inhibitory activity of 2cm and 3.2cm at 10-1 and 10-5 dilution respectively. Like that Dabur (A3) showed activity of 2cm and 3cm for Pseudomonas aeruginosa at 10-1 and 10-5 dilutions respectively. The clinical pathogens Enterococcus faecium and Staphylococcus aureus showed an increase in antibiotic sensitivity with an increase in dilution from10-1and 10-5. The antibiotic activity of Natural Kasaragod honey (N1) against different dilutions of Enterococcus faecium and Staphylococcus aureus. It was observed that the strain Staphylococcus aureus showed high sensitivity to all honey samples. Among the table increase in antibiotic activity with an increase in dilution of samples from 10-1 to 10-5. The antibiotic activity of Aralam honey (N2) against different dilutions of Enterococcus faecium and Staphylococcus aureus. It was observed that the highest antibiotic sensitivity was at 10 -5 least activity was at 10-1 which means as dilution increases antibiotic sensitivity also increased. Antibiotic activity of Jamun honey (N3) against the diluted culture of Enterococcus faecium and Staphylococcus aureus, which showed highest antibiotic sensitivity at 10-5 (2.0 cm) and least activity at 10-1 (0.7cm), which means as dilution increases antibiotic sensitivity also increased. The antibiotic activity of Lion honey (A1) against diluted culture of Enterococcus faecium and Staphylococcus aureus. It was observed that the highest antibiotic sensitivity was at 10-5 and the least activity was at 10¹, which means as dilution increases antibiotic sensitivity also increases. Antibiotic activity of Be Good honey (A2) against the diluted culture of Enterococcus faecium and Staphylococcus aureus, which showed the highest antibiotic sensitivity at 10-5 and the least activity at 10-¹, which means as dilution increases antibiotic sensitivity also increased. The antibiotic activity of Dabur honey(A3) against the diluted culture of Enterococcus faecium and Staphylococcus aureus, showed the highest antibiotic sensitivity at 10-5 and the least activity at 10-¹, which means as dilution increases antibiotic sensitivity also increased. An increase in antibiotic sensitivity along the dilution of broth, and there was a decrease in many bacterial colonies along the graph. From Figure 2a, the antibiotic activity of Natural (N1) honey against E.coli increases with an increase in dilution of broth, and the number of colonies decreases with an increase in dilution. E.coli is less inhibited by (N1) honey at 10-1 concentration with 1.3 cm. Thus antibacterial activity of Natural honey was high at 10-5 concentration which was 2.4 cm, and the number of bacterial colonies was less at 10-5 concentration which was 40x105 (CFU/μl). The Figure shows the increasing activity along the concentration. It shows less antibiotic sensitivity of 3.8cm at 10-1 concentration.
In Figure 2a, Escherichia coli was highly inhibited by Aralam (N2) honey with antibiotic sensitivity of 4.5 cm at 10-5 concentration, which shows the high inhibition zone diameter among all honey samples. Thus the number of colonies present decreases along the concentrations, which shows a high number of colonies (365x10) at 10-¹ dilution and less number of colonies (40x105) at 10-5. It reveals the antibiotic activity of Jamun (N3) honey against Escherichia coli. The graph showed different antibiotic sensitivity from dilution 10-1 to 10-5 with 3.3, 3.5, 3.8, 4.1 and 4.4 cm. Jamun (N3) honey was effective against E. coli. Hence, the inhibition zone diameter of Jamun (N3) honey against E.coli was high at 10– with
4.4 cm. Tables 2a and 2b, represented the antibiotic activity of Commercial (Processed) honey samples Lion (A1), Be Good (A2), and Dabur (A3) against Escherichia coli. It showed an increase in antibiotic sensitivity along the different concentrations of broth, and there was a decrease in several bacterial colonies along the graph. From Figure 2a, the antibiotic activity of Lion (A1) honey against E.coli increases with an increase in dilution of broth, and the number of colonies decreases with an increase in dilution. E.coli is less inhibited by Lion honey at 10-1, 10-2, and 10-3 concentration with 3.8 cm. Thus antibacterial activity of Lion honey was high at 10-5 concentration which was 4.3 cm, and the number of bacterial colonies was less at 10-5 concentration which was 40x105(CFU/μl). It showed the increasing activity along the concentration.
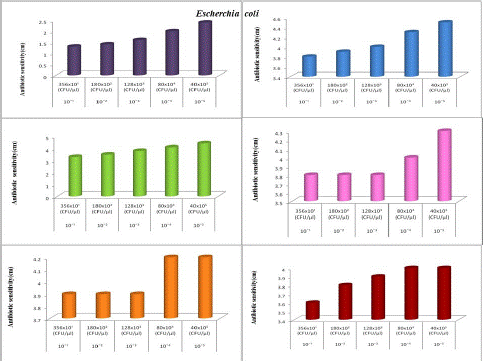
Figure 2a: The antibiotic activity of unprocessed honey, Natural honey Kasaragod (N1), Aralam (N2), Jamun (A3) and processed honey, Lion (A1), Be good (A2), Dabur (A3) honey samples against clinical pathogen Escherichia coli,
From Figure 2a, Escherichia coli was inhibited by Be good (A2) honey with antibiotic sensitivity of 3.9 and 4.2 cm at dilution from 10-1 to 10-5 concentration. Thus the number of colonies present decreases along increasing dilution, in which a high number of colonies (365x10) present at 10-1 dilution and less number of colonies (40x105) at 10-5. It revealed the antibiotic activity of Dabur (A3) honey against Escherichia coli. The graph showed different antibiotic sensitivity from dilution 10–¹ to 10–5 with 3.6, 3.8, 3.9, 4, and 4 cm. Dabur (A3) honey was effective against E.coli. Hence, the inhibition zone diameter of Dabur (A3) honey against E.coli was high at 10-4 and 10-5 of 80x104 (CFU/μl) and 40x105 with 4 cm. Figure 2b and Table 2 represented the antibiotic activity of Natural (Unprocessed) honey samples Natural (N1), Kasaragod, Aralam (N2), Jamun (N3) against Enterobacter cloacae. It showed that increase in antibiotic sensitivity along the dilutions of broth, and there was a decrease in many bacterial colonies along the graph.
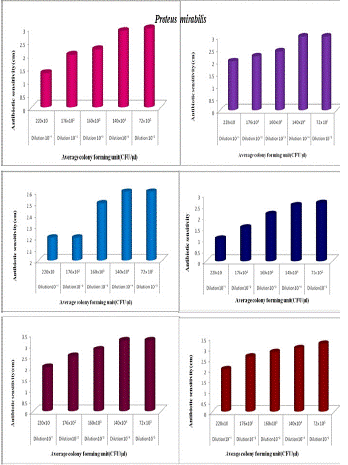
Figure 2b: The antibiotic activity of unprocessed honey, Natural honey Kasaragod (N1), Aralam (N2), Jamun (A3) and processed honey, Lion (A1), Be good (A2), Dabur (A3) honey samples against clinical pathogen Enterobacter cloacae
From Figure 2b, the antibacterial activity of Natural (N1) honey against Enterobacter cloacae increases with an increase in dilution of broth, and the number of colonies decreases with an increase in dilution. Enterobacter cloacae is less inhibited by (N1) honey at 10-1 concentration which is 1cm. Thus antibiotic activity of Natural honey was high at 10-4 and 10-5 concentrations which was 1.7 cm, and the number of bacterial colonies was less at 10-5 concentration which was 32x105(CFU/μl). Figure 2b, showed the increasing activity along the dilutions. From the figure, the Enterobacter cloacae were moderately inhibited by Aralam (N2) honey with antibiotic sensitivity 1.2, 1.8, 2.5, 2.6, and 2.7 cm at concentrations 10-1 to 10-5. It reveals that Enterobacter cloacae is highly inhibited by Aralam (N2) honey at 10-5 concentration, which shows 2.7 cm. Thus the number of colonies present decreases along increasing dilution, in which a high number of colonies (365x10) present at 10–¹ dilution and less number of colonies (40x105) at 10-5. It also revealed the antimicrobial effect of Jamun (N3) honey against Enterobacter cloacae. The graph shows different antibiotic sensitivity from dilution 10-1 to 10-5 with 1, 2.2, 2.3, 2.5, and 2.7 cm. Jamun (N3) honey was effective against Enterobacter cloace. Hence, the inhibition zone diameter of Jamun (N3) honey against Enterobacter cloacae was high at 10–5 with 2.7 cm.
The antibiotic activity of Commercial (Processed) honey samples Lion (A1), Be Good (A2), and Dabur (A3) against Enterobacter cloacae. It showed an increase in antibiotic sensitivity along with the decreasing concentration of broth, and there was a decrease in several bacterial colonies along the graph. The antibiotic activity of Lion (A1) honey against Enterobacter cloacae increases with an increase in dilution of broth and the number of colonies decreases with an increase in dilution. Enterobacter cloacae is less inhibited by Lion honey at 10-1 concentration. Thus antibacterial activity of Natural honey was high at 10-5 concentration which was 3.5 cm, and the number of bacterial colonies was less at 10–5 concentration which was 32x105(CFU/μl). It showed increasing activity along with the increase in dilution. The Enterobacter cloacae were less inhibited by Be good (A2) honey with antibiotic sensitivity 1 cm at 10–¹ concentration, which shows the less inhibition zone diameter among all honey samples. Thus the number of colonies present decreases along increasing concentration, in which more number of colonies (252x10 CFU/μl) present at 10–¹ dilution and less number of colonies (32x105) at 10-5. It revealed the antibiotic activity of Dabur (A3) honey against Enterobacter cloacae. The graph shows different antibiotic sensitivity from dilution 10-1 to 10-5 with 1.5, 2.6, 2.8, 3.3, and 3.9 cm. Dabur (A3) honey was effective against Enterobacter cloacae. Hence, the inhibition zone diameter of Dabur (A3) honey against Enterobacter coli was high at 10-5 with 3.9 cm. Figure 2c shows the antibiotic activity of natural (N1) honey at different dilutions against Pseudomonas aeruginosa. At 10-1 dilution number of organisms is higher and antibiotic activity is lower (1.4cm). At 10-5 dilution the number of organisms decreased and antibiotic activity increased (3.6cm) as shown in the Figure. It also showed the antibiotic activity of natural (N2) honey at different dilutions against Pseudomonas aeruginosa. At 10-1 dilution, antibiotic activity is lower (2cm) and at 10-5 dilution antibiotic activity is increased (3.4cm). It showed the antibiotic activity of Jamun (N3) honey at different dilutions against Pseudomonas aeruginosa. At 10-1 antibiotic activity is lower (2cm) and at 10-5 dilution antibiotic activity increased (3.3cm) with a decrease in several organisms. It showed the antibiotic activity of Lion (A1) honey at different dilutions against Pseudomonas aeruginosa. At 10-1 dilution number of organisms is higher and antibiotic activity is lower (2cm). At 10-5 dilution the number of organisms decreased and antibiotic activity increased (3cm). Figure 2c shows the antibiotic activity of Begood (A2) honey at different dilutions against Pseudomonas aeruginosa. At 10-1 dilution number of organisms is more and antibiotic activity is less (1.5cm). At 10-5 antibiotic activity increased (2.6cm). It showed the antibiotic activity of Dabur (A3) honey at different dilutions against Pseudomonas aeruginosa. At 10-1 dilution number of organisms is higher and antibiotic activity is lower (2cm). At 10-5 dilution the number of organisms decreased and antibiotic activity increased (3cm).
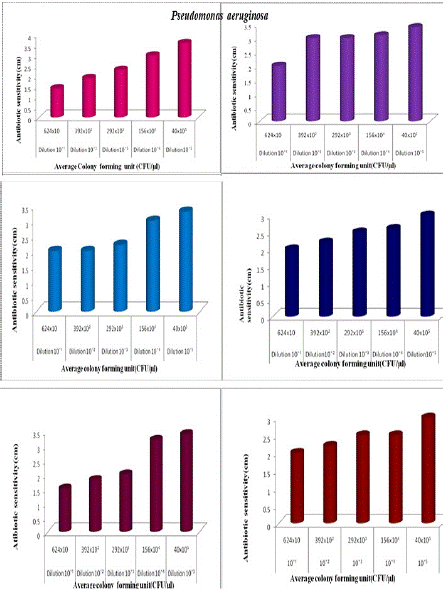
Figure 2c: The antibiotic activity of unprocessed honey, Natural honey Kasaragod (N1), Aralam (N2), Jamun (A3) and processed honey, Lion(A1), Be good (A2), Dabur(A3) honey samples against clinical pathogen Pseudomonas aeruginosa,
Figure 2d shows the antibiotic activity of natural (N1) honey at different dilutions against Proteus mirabilis. At 10-1 dilution number of organisms is higher and antibiotic activity is lower (1.3cm).At 10-5 dilution the number of organisms decreased and antibiotic activity increased (3cm). Figure 2d shows the antibiotic activity of Aralam (N2) honey at different dilutions against Proteus mirabilis. At 10-1 dilution number of organisms is higher and antibiotic activity is lower (2cm). At 10-5 dilution, antibiotic activity is increased (3cm). It showed the antibiotic activity of Jamun (N3) honey at different dilutions against Proteus mirabilis. At 10-1 dilution number of organisms is higher and antibiotic activity is lower (2.2cm). At 10-5 dilution the number of organisms decreased and antibiotic activity is increased (2.6cm). It showed the antibiotic activity of Lion (A1) honey at different dilutions against Proteus mirabilis. At 10-1 dilution number of organisms is higher and antibiotic activity is lower (1.0cm).At 10-5 dilution the number of organisms decreased and antibiotic activity is increased (2.6cm). It showed the antibiotic activity of Begood (A2) honey at different dilutions against Proteus mirabilis. At 10-1 dilution number of organism is higher and antibiotic activity is lower (2cm).At 10-5 dilution antibiotic activity is increased (3.2cm). The Figure showed the antibiotic activity of Dabur (A3) honey at different dilutions against Proteus mirabilis. At 10-1 dilution number of organism is higher and antibiotic activity is lower (2.0cm). At 10-5 dilution the number of organism decreased and antibiotic activity is increased (3.2cm). From the above figure.2e, revealed the antibacterial activity of unprocessed (Natural honey Kasaragod (N1), Aralam (N2), Jamun (N3) and processed Lion (A1), Begood (A2), Dabur (A3) against Entercoccus faecium. It showed the antibiotic activity of different honey samples towards Enterococcus faecium. The higher antibiotic sensitivity was observed in Dabur (A2) honey was 2.5cm and lower activity was observed in natural honey Kasaragod (N1) was 1.2cm. The Figure 2e, represented the antibiotic sensitivity of Natural honey against at different dilution of Enterococcus faecium. From the graph, it was observed that increase in antibiotic activity when increase in dilution. Natural honey Kasaragod showed highest antibiotic sensitivity at 10-5and least activity at 10-¹. Antibiotic sensitivity of Aralam (N2) honey against at different dilution of Enterococcus faecium was represented in figure 2e. The graph showed that the sensitivity was gradually increased with increase in dilution. The higher sensitivity was 2.5cm at dilution 10-5 and lower sensitivity was 1.5cm at dilution 10-¹. Antibiotic activity of Jamun honey against at different dilution of Enterococcus faecium was represented in the figure. The graph showed increase in sensitivity of Jamun honey against Enterococcus faecium. In dilution 10-¹ the sensitivity was 0.7cm and in dilution 10-5 the sensitivity was 2cm. The Figure2e, represented the antibiotic sensitivity of Lion honey against at different dilution of Enterococcus faecium. From the graph, it was observed that increase in antibiotic activity when increase in dilution. Lion honey showed highest antibiotic sensitivity at 10-5and least activity at 10-¹. The antibiotic sensitivity of Be good honey against at different dilution of Enterococcus faecium was represented in figure. The graph showed that the sensitivity was gradually increased with increase in dilution. The higher sensitivity was 3 at dilution 10-5and lower sensitivity was 1.2 at dilution 10-1. The antibiotic activity of Dabur honey against at different dilution of Enterococcus faecium was represented in figure. The graph showed increase in sensitivity of Dabur honey against Enterococcus faecium. In dilution 10-1 the sensitivity was 0.9cm and in dilution 10-5the sensitivity was 2.7cm. The above figure 2f, showed that the antibiotic activity of different honey samples towards Staphylococcus aureus. The higher antibiotic sensitivity was observed in Dabur (A3) honey was 3.5cm and lower activity was observed in natural honey Kasaragod (N1) was found to be 1.6cm zone formation. The Figure 2f, represented the antibiotic sensitivity of Natural honey Kasaragod against at different dilution of Staphylococcus aureus. From the graph, it was observed that increase in antibiotic activity when increase in dilution. Natural honey Kasaragod showed highest antibiotic sensitivity at 10-5 and least activity at 10-1. The antibiotic sensitivity of Aralam honey against at different dilution of was Staphylococcus aureus represented in the figure. The graph showed that the sensitivity was gradually increased with increase in dilution. The higher sensitivity was 3.5 at dilution 10-5 and lower sensitivity was 2 at dilution 10-1. Antibiotic activity of Jamun honey against at different dilution of Staphylococcus aureus was represented in figure. The graph showed increase in sensitivity of Jamun honey against Staphylococcus aureus in dilution 10-1 the sensitivity was 1.5cm and in dilution 10-5 the sensitivity was found to be 3cm. The Figure, represents the antibiotic sensitivity of Lion honey against at different dilution of Staphylococcus aureus. From the graph, it was observed that increase in antibiotic activity when increase in dilution. Lion honey showed highest antibiotic sensitivity at 10-5 and least activity at 10-¹. The Antibiotic sensitivity of Begood honey against at different dilution of Staphylococcus aureus was represented in figure. The graph showed that the sensitivity was gradually increased with increase in dilution. The higher sensitivity was 4.5cm at dilution 10-5 and lower sensitivity was 1.5cm at dilution 10-1. There is a large difference between 10-¹ and 10-5 (3cm). The antibiotic activity of Dabur honey against at different dilution of Staphylococcus aureus was represented in the figure. The graph showed an increase in the sensitivity of Dabur honey against Staphylococcus aureus in dilution 10-¹ the sensitivity was 1.5cm and in dilution 10-5 the sensitivity was observed to be 3.4cm. In the case of Enterobacter cloacae, the antibiotic activity of Natural (N1) honey Kasaragod against the undiluted culture of 260x10³(CFU/μl) was 1.2cm, and that of diluted culture of 252x10 (CFU/μl) gives 1cm which shows difference of 0.2cm. Aralam (N2) honey inhibits the growth of undiluted Enterobacter cloacae which gives antibiotic sensitivity of 1.4cm and gives antibiotic sensitivity of 1.2cm in diluted culture. It shows a 0.2 cm variation between them. Jamun (N3) honey showed antibiotic sensitivity of 2.1cm against undiluted culture, whereas N3 showed 1cm of antibiotic sensitivity against diluted culture, the difference between antibiotic sensitivity in diluted and undiluted culture was 1.1cm. In Lion (A1), Be Good (A2), and Dabur (A3) honey samples inhibit the growth of undiluted culture of Enterobacter cloacae, which showed antibiotic sensitivity of 2.2, 2.5 cm. In diluted culture, honey samples inhibit growth with antibiotic sensitivity of 2.8, 2.2, and 1.5cm. They showed differences of 0.6, 0.3, and 1cm. Here diluted culture of Enterobacter cloacae was highly inhibited by all honey samples than the undiluted culture. In the case of Natural Kasaragod (N1) honey against Pseudomonas aeruginosa, the rate of antibiotic sensitivity in undiluted culture is
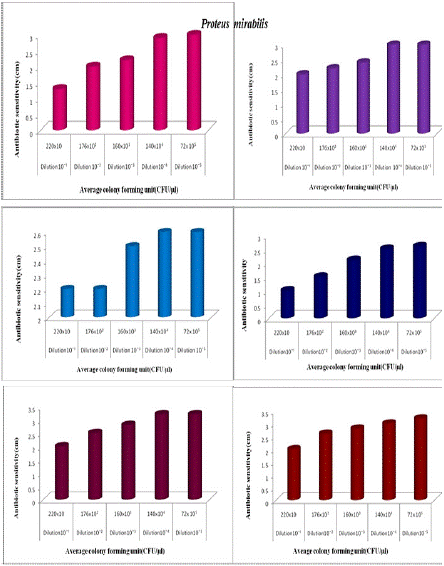
Figure 2d: The antibiotic activity of unprocessed honey, (Natural honey Kasaragod (N1), Aralam (N2), Jamun (A3) and processed honey, Lion(A1), Be good (A2), Dabur(A3) honey samples against clinical pathogen Proteus mirabilis,
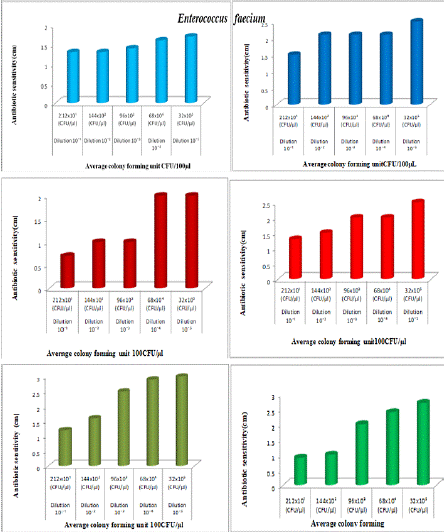
Figure 2e: The antibiotic activity of unprocessed honey, Natural honey Kasaragod (N1), Aralam (N2), Jamun (A3) and processed honey, Lion(A1), Be good (A2), Dabur(A3) honey samples against clinical pathogen Enterococcus faecium,
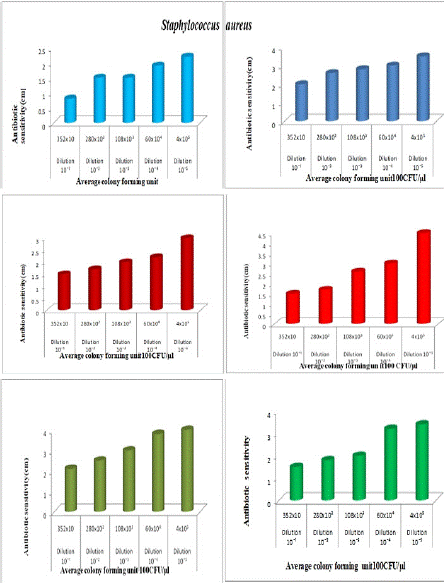
Figure 2f: The antibiotic activity of unprocessed honey, Natural honey Kasaragod (N1), Aralam (N2), Jamun (A3) and processed honey, Lion (A1), Be good (A2), Dabur(A3) honey samples against clinical pathogen Staphylococcus aureus
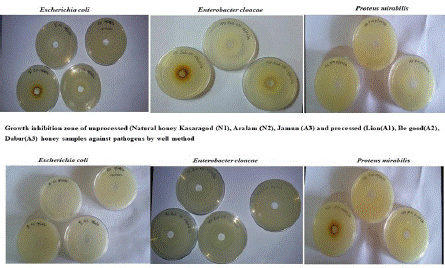
Figure 3a: Antibiotic activity Agar plate assay by well diffusion method.
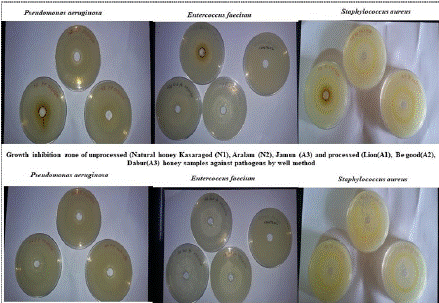
Figure 3b: Antibiotic activity Agar plate assay by well diffusion method.
1.2cm and that of diluted culture is 1.4 cm. In the case of Aralam (N2) honey, the rate of antibiotic sensitivity in undiluted culture is 2.4cm and that of diluted culture is 2 cm. In the case of Jamun (N3) honey, the rate of antibiotic sensitivity in undiluted culture is 2.4 cm, and that of diluted culture is 2 cm. In the case of Lion (A1) honey, the rate of antibiotic sensitivity of undiluted culture is 2.5cm and that of diluted culture is 2 cm.
This means in these four samples there is a slight variation (0.2cm -0.5cm) in the antibiotic sensitivity between diluted and undiluted cultures. In the case of Begood (A2) honey, the rate of antibiotic sensitivity of undiluted culture is 3 and that of diluted culture is 1.5 cm. In the case of Dabur (A3) honey, the rate of antibiotic sensitivity in undiluted culture is 3.2 cm and that of diluted culture is 2 cm, which means higher variation (1.2 cm-1.5 cm) in the antibiotic sensitivity between diluted and undiluted culture. In the case of Natural Kasaragod (N1) honey against Proteus mirabilis, the rate of antibiotic sensitivity for undiluted culture is 1.5 and that of diluted culture is 1.3 cm.
In the case of Aralam (N2) honey rate of antibiotic sensitivity for undiluted culture is 2.4 cm and that of diluted culture is 2 cm. In the case of Jamun (N3) honey rate of antibiotic sensitivity for undiluted culture is 2.6 cm and that of diluted culture is 2.2 cm. That means all these three samples show slight variation (0.2 cm-0.4 cm) in the antibiotic sensitivity between diluted and undiluted cultures. In the case of Be good (A2) honey rate of antibiotic sensitivity for undiluted culture is 2.8 cm and that of diluted culture is 2 cm.
In the case of Dabur (A3) honey rate of antibiotic sensitivity for undiluted culture is 3 cm and that of diluted culture is 2 cm, which means both A2 and A3 show much higher variation(0.8 cm-1 cm) in antibiotic sensitivity. In the case of Lion (A1) honey rate of antibiotic sensitivity for undiluted culture is 2.8 cm and that of diluted culture is 1 cm, which means A1 shows a higher variation (1.8 cm) in the antibiotic sensitivity between diluted and undiluted culture.
Tables 2a and 2b, showed the antibiotic activity of different honey samples N1, N2, N3 (Natural) and A1, A2, A3 (Artificial) against undiluted and diluted cultures of Enterococcus faecium and Staphylococcus aureus. In the case of Natural honey (N1) against Enterococcus faecium, the rate of antibiotic sensitivity of undiluted culture was 1.2 cm, and that of diluted culture was 1.3 cm. In the case of Aralam honey (N2); the rate of antibiotic sensitivity in undiluted culture was 1.4 and that of diluted culture was 1.5cm. In the case of Jamun Honey (N3); the rate of antibiotic sensitivity against undiluted culture was 1.6 and that of diluted culture was .07cm. This means in these three unprocessed honey samples there is a slight variation (0.1-0.9) in the antibiotic sensitivity between diluted and undiluted cultures. In the case of Lion Honey (A1); the rate of antibiotic sensitivity in undiluted culture was
2.4cm and that of diluted culture was 1.3cm, In the case of Begood Honey (A2); the rate of antibiotic sensitivity in undiluted culture is 2.4cm and that of diluted culture is 1.2cm, In case of Dabur Honey (A3); the rate of antibiotic sensitivity undiluted culture is 2.5cm and that of diluted culture is 0.92cm. This means higher variation (1.1-1.6) in the antibiotic sensitivity between diluted and undiluted cultures. In the case of Natural honey, the rate of antibiotic sensitivity of undiluted culture was 1.6cm, and that of diluted was 0.8cm, In the case of Aralam (N2) honey, the rate of antibiotic sensitivity of undiluted culture was 2.9cm, and that of diluted was 0.9cm, In case of Jamun (N3) honey, the rate of antibiotic sensitivity undiluted culture was 3cm and that of diluted was 1.5cm.
That means all these three samples show a small variation (0.8-1) in the antibiotic sensitivity between diluted and undiluted cultures. In the case of Lion (A2), honey the rate of antibiotic sensitivity in diluted culture was 3.1cm, and that of diluted was 1.5cm. In the case of Be good (A2) honey, the rate of antibiotic sensitivity in undiluted culture was 3.5cm and that of diluted was 1cm. In the case of Dabur (A3) honey, the rate of antibiotic sensitivity of undiluted culture was 3.5cm and that of diluted was 1.5cm, which means both A1 and A2 showed much higher variation (1.5-1.6) in antibiotic sensitivity. In the case of Dabur (A3) honey, the rate of antibiotic sensitivity for undiluted culture is 3.5cm, and that of diluted culture is 1.5cm, which means A3 shows a higher variation (2) in the antibiotic sensitivity between diluted and undiluted culture.
Spectral Analysis
The honey samples were diluted in ethanol and deionized water and allowed for centrifugation at 5000 rpm for 4 hours. These catalyzed samples were subjected to spectral analysis and the presence of active ingredients or compounds was detected by UV- Vis spectrometer.
UV Spectral Analysis of Honey: UV Spectra Data Acquisition
The Spectral analysis of different honey samples undiluted (Figures 4a and 4b) and diluted in Ethanol (Figures 4c and 4d) were observed. From the spectral analysis (Figures 4a,4b,4c, and 4d), it was confirmed that the presence of active ingredients or compounds were present in the tested honey samples, which are responsible for the antibiotic activity against the selected clinical pathogens of Escherichia coli, Enterobacter cloacae, Pseudomonas aeruginosa, Proteus mirabilis, Enterococcus faecium, and Staphylococcus aureus. Figures 4a- 4d represented the spectrum of Natural (Natural honey, Kasaragod (N1), Aralam (N2), Jamun (N3) honey and Commercial (Lion (A1), Be good (A2), Dabur (A3) honey. Honey is a dense substance, so its transparency is less, which means more absorbing capacity. The transparency of honey is less; hence its absorbance is high. The particles present in honey absorb light and emit partial light. In the graph Nanometer (nm) shows a particular compound present in honey samples, and that absorbance shows the density of the honey samples. In Figure 4a, the spectrum of Natural honey (N1, N2, and N3) gives slight differences in peaks with wavelength 265nm and 270 nm. Peaks showed one compound the corresponding absorbance shows density. Here Natural (N1) honey Kasaragod is taken as the standard value because of the absence of other additives or preservatives, which shows high purity with a wavelength of 265nm. In the N2 graph, peaks show a wavelength of 270 nm with an optical density (OD) value of 0.899 OD. The N3 graph gives a peak with a wavelength the same as that of N2 (270 nm) against a 1.677 OD value. Here, N1, N2, and N3 showed the same wavelength but a difference in OD value which represents the different concentrations of compound present in honey samples, ie., from the absorbance value it fluctuates and the density may differ. In Figure 4b, the Spectrum of commercial honey (A1, A2, and A3) shows peaks with the same wavelength of 270 nm for Lion (A1), Be good (A2), and Dabur (A3) honey samples. Here the absorbance value fluctuates and gets different densities. The graph of Lion (A1) honey gives a wavelength of the same nanometer (270 nm) and an absorbance value of 0.0476 OD. In graph A2 (Be good), a peak with 270 nm wavelength gives an absorbance of 1.707 OD. The graph of Dabur (A3) also has a peak that gives the same wavelength of 270 nm and a different absorbance value of 1.896 OD.
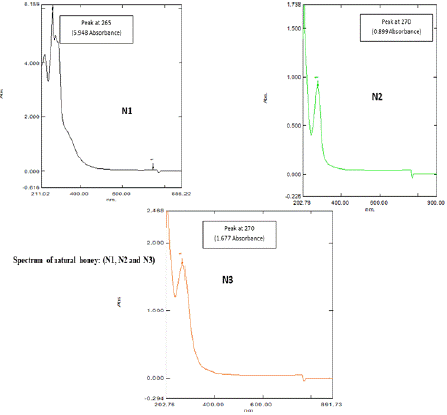
Figure 4a: UV Spectrum of Natural honey: (N1, N2 and N3).
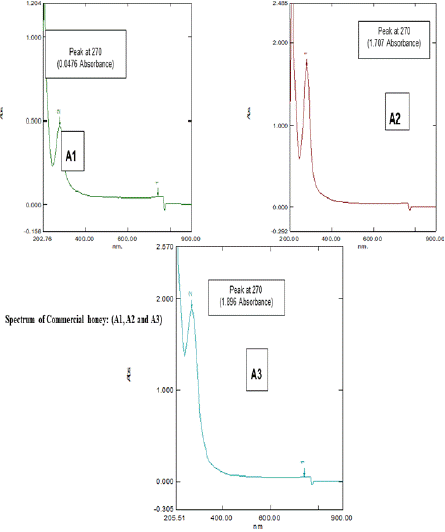
Figure 4b: UV Spectrum of Commercial honey: (A1, A2 and A3).
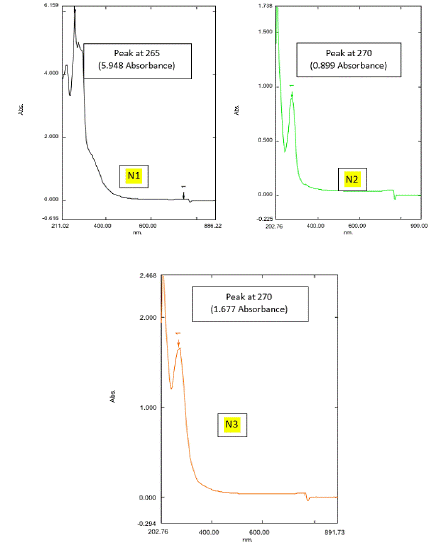
Figure 4c: UV Spectrum of natural honey: (N1, N2 and N3).
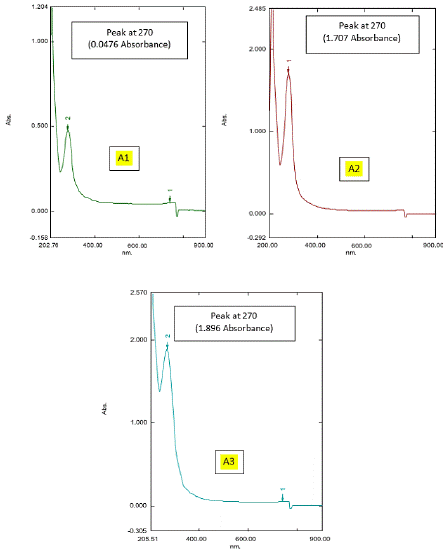
Figure 4d: UV Spectrum of Commercial honey: (A1, A2 and A3)
In Figure 4c, among the Natural honey samples (N1, N2, and N3) Natura (N1) honey, Kasaragod gives a high absorbance value that of Aralam (N2) and Jamun (N3) honey, with 948 OD, which means natural (N1) honey, Kasaragod shows high density with an original concentration of the compound which absorbs the light more. In Figure 4d, among the Commercial honey samples (A1, A2, and A3), Dabur (A3) shows a high optical density that of Lion (A1) and Be Good (A2) honey, with 1.896 OD. Lion (A1) shows a lower absorbance value of 0.0476 OD. Here the absorbance value of 1.896 OD of the Dabur (A3) sample gives more density. Thus, it consists of a pure denser concentration of the compounds which absorbs more light. From figures 4a-4d, among all honey samples of natural and commercial, Natural (N1) honey, Kasaragod shows a high absorbance value of 5.948 OD. Hence, it shows more density which means the pure one. The result was sustained by several preceding researches which have established those various kinds of honey, together commercially and locally manufactured, have antibacterial activity. Nzeako and Hamdi (2000) reported in their work on six commercial honeys that inhibition of S. aureus, E. coli, and P. aeruginosa also did not occur at honey concentrations of 40%. In comparison to the current work, these authors also found that honey inhibited Candida albicans, while the zone of inhibition was less related to other organisms. Ceyhan and Ugar (2001) studied 84 honeys against eight bacteria and two fungi and showed that honey has broad-spectrum activity. Moreover, these authors stated that the antibacterial activity of honey was greater than that which could be attributed to the sugar content of the honey. The antibacterial activity of honey has also been examined for its possible use in reducing food-borne pathogens [35], avoiding catheter exit or entry site infection [25], for the cure of colitis [3] or even to guard the gastric mucous in H. pylori encouraged inflammation [2,25]. The claim of honey to wounds to animals in veterinary environments has also been renowned [18].
From the spectral analysis, two characteristic peaks were observed at around 270 nm and 300 nm, those are associated with the absorbance of benzoic, salicylic, and aryl-aliphatic acids in honey. The shape of the spectral curves was quite related, exclusively between 250–400 nm, with piercing variances in peak absorbance intensity at 270nm and 300 nm. These spectral results are consistent with previously reported work [10]. The UV–Vis absorption spectra of sixteen bulk Tuscany honey samples were reported similarly, including acacia, clover, etc [26]. Different peak absorbance intensities were reported around 270nm–280 nm depending on the type of honey. The minor peaks were also reported between 300nm–335 nm. The characteristic feature of original or raw UV spectral data is high noise with very high absorbance, particularly in the interim of 190nm – 250nm.
This raw spectral data is in unrelated information such as background information and systematic noise coming from the influences of light scattering, differences in path length, sample particle size, short lamp intensity at the jump of spectral acquisition and other features [39]. Consequently, to attain a satisfactory result, in this study for advance chemo metrics calculation used comparatively low noise spectral data using pre-processed spectral data in the interval of 250nm–400 nm.
Antimicrobial agents are principally significant in reducing the global burden of infectious diseases. However, as resilient pathogens grow and extend, the effectiveness of the antibiotics is reduced. This kind of bacterial resistance to antimicrobial agents poses a precise severe threat to public health. Hence, substitute antimicrobial strategies are directly needed, and thus this condition led to a re-evaluation of the therapeutic use of ancient remedies, such as honey. Honey has been used since ancient times as a method of accelerating wound healing and as an agent for the treatment of ulcers and other skin infections resulting from burns and wounds. The therapeutic properties of honey can be recognized because it deals with antibacterial activity, sustains a moist wound environment that promotes healing, and has a high viscosity which helps to offer a defensive barrier to avoid infection. The practice of drinking milk is quite common in our day-to-day activities. While using raw milk or even after boiling some of the pathogens remain in it and lead to some common infection either directly or indirectly. So this topic is selected to identify the antibacterial activity of honey and to inhibit the growth of these pathogens using honey as a natural antibiotic compound. For this study, three samples of Natural (unprocessed) and three samples of Commercial (processed) were collected from different sources in the Kasaragod district. Cultures of pathogenic bacteria Escherichia coli, Enterobacter cloacae, Pseudomonas aeruginosa, Proteus mirabilis, Enterococcus faecium, and Staphylococcus aureus were obtained from Kanhangad Diagnostic Centre (KDC) Lab, Kanhangad. Obtained cultures were sub-cultured on Nutrient agar and incubated aerobically at 37°c. The organism which was maintained in the laboratory on nutrient broth underwent serial dilution and the concentration of the pathogen at different dilutions was examined.
To study the antibiotic activity of honey against Escherichia coli, Enterobacter cloacae, Pseudomonas aeruginosa, Proteus mirabilis, Enterococcus faecium, and Staphylococcus aureus, well diffusion assay method was used. The results were obtained by measuring the zones around the wells after the diminution of the well size. Antibiotic activity of each honey sample obtained against both undiluted and diluted cultures of clinical pathogens E.coli, Enterobacter cloacae, Pseudomonas aeruginosa, Proteus mirabilis, Enterococcus faecium, and Staphylococcus aureus. It yielded positive results on undiluted and diluted cultures using all honey samples. The growth of the undiluted culture was less inhibited by different honey samples and the diluted was highly inhibited by all honey samples, and the number of bacterial colonies was high in the undiluted culture whereas less in the diluted culture. The results showed the different antibiotic activity of different honey samples against undiluted and diluted cultures of E.coli and Enterobacter cloacae, in which antibiotic activity of Lion (A2) honey against diluted culture shows high activity and antibiotic activity of Natural (N1) honey against undiluted culture of shows less activity. In 10-1 dilution, the number of organisms was more and the diameter of zone formation was less. In 10-5 dilution, the number of organisms was less and the diameter of zone formation was more. Hence, it revealed that as dilution increased, antibiotic sensitivity also increased. Thus, to reduce the cost, and burden of infectious diseases and to inhibit the growth of pathogens from milk, these suitable methods could be used more often in the future.
Conclusion
In conclusion, Microbial resistance to honey has never been reported, which makes it a very promising topical antimicrobial agent against the infection of antibiotic-resistant bacteria and in the treatment of long-lasting wound infections that do not react to antibiotic remedies. Therefore honey has been cast off as a former option for medication. The exact explanation of honey is not known, but it is clear that the higher the concentration of honey the greater its usefulness as an antibacterial agent. It is too obvious that the antibacterial effect declines over time and that different species of bacteria vary in their susceptibility to honey.
Although there is evidence of antibacterial activity from the use of honey in the topical treatment of infected wounds, further consideration needs to be given to its parenteral application and healing possessions to optimize the use of this product in medical and universal infections.
The result showed that the antibiotic activity of all honey samples against diluted cultures was higher than that of undiluted cultures. Hence, the antibiotic activity of all samples against the diluted culture was observed to be higher than that against the undiluted culture because of less number of colonies present in the diluted culture. Thus antibiotic sensitivity increases with an increase in dilution and a decrease in several colonies or cells. The results revealed that the possibility of using these honey samples for the inhibition of bacterial strains Escherichia coli, Enterobacter cloacae, Pseudomonas aeruginosa, Proteus mirabilis, Enterococcus faecium, and Staphylococcus aureus which is present in raw milk and wounds cause infectious diseases. The honey samples capable of antibiotic activity against clinical pathogens have the potential to be used as an effective tool for inhibiting the growth of pathogenic microorganisms. Furthermore, results indicated that the honey samples could be potentially used in the inhibition of bacterial microorganisms that cause infectious diseases and had a promising application as an antibacterial agent against pathogens of raw milk. In General, the antibacterial activity of honey has been confirmed though there are distinct results among investigators as to whether concentration is effective or not. Perfectly, these characteristics are due to more than one factorial influence. Hence, further research is compulsory in this area. Furthermore, the world nowadays desires advanced assessments of natural bioactive compounds that can be applied against pathogens and to combat microorganisms with negligible adjacent effects or significances of over dosage.
References
- Al-Nahari AA, Almasaudi SB, Sm AE-G, Barbour E, Al Jaouni SK, Harakeh S. Antimicrobial activities of Saudi honey against Pseudomonas aeruginosa. Saudi J Biol Sci. 2015; 22: 521–5.
- Ali MATM. Prevention of ammonia-induced gastric lesions in rats by natural honey. Journal of nutritional and environmental Medicine. 2003; 13: 239–46.
- Bilsel Y, Bugra D, Yamaner S, Bulut T, Cevikbas U, Turkoglu U. Could honey have a place in colitis therapy? Effects of honey, prednisolone, and disulfiram on inflammation, nitric oxide, and free radical formation. Digestive Surgery. 2002; 19: 306-11.
- Bogdanov S, Jurendic T, Sieber R, Gallmann P. Honey for nutrition and health: A review. Journal of the American College of Nutrition. 2008; 27: 677–89.
- Ceyhan N, Ugar A. Investigation of in vitro antimicrobial activity of honey. Rivista Di Biologia-Biology Forum. 2001; 94: 363-71.
- Chikhaoui M, Hajjouji EL, Belghiti A, Badril W, Moouslin JJ. Antibacterial activity of honey on some antibiotic-resistant strains, systematic review. Arabian Journal of Medicinal and Aromatic Plants. 2023; 9: 165–83.
- Chute RK, N.g D, Kawale M. Antimicrobial activity of Indian honey against clinical Isolates. Asiatic Journal of Biotechnology Res. 2010; 1: 35–8.
- Cooper RA, Molan PC, Harding KG. Antibacterial activity of honey against strains of Staphylococcus aureus from infected wounds. Journal of the royal society of medicine. 1999; 92: 283-5.
- Cooper RA, Molan PC, Harding KG. The sensitivity to honey of Gram- positive cocci of clinical significance isolated from wounds. Journal of Applied Microbiology. 2002; 93: 857–63.
- Dimitrova B, Gevrenova R, Anklam E. Analysis of phenolic acids in honeys of different floral origin by solid-pase extraction and high-performance liquid chromatography. Phytochem Anal. 2006; 18: 24–32.
- H DJ. Antibacterial effect of honey. Apiacta. 1989; 14: 7.
- Gheldof N, Wang XH, Engeseth NJ. Identification and quantification of antioxidant components of honeys from various floral sources. J Agric Food Chem. 2002; 50: 5870–7.
- Irish J, Blair S, Carter DA. The antibacterial activity of honey derived from Australian flora. PLoS One. 2011; 6: e18229.
- Kwakman PHS, te Velde AA, de Boer L, Speijer D, Vanden broucke-Grauls CMJE, Zaat SAJ. How honey kills bacteria FASEB Journal. 2010; 24: 2576–82.
- Lu J, Turnbull L, Burke MC, Liu M, Carter DA, Schlothauer RC, et al. Manuka-type honeys can eradicate biofilms produced by Staphylococcus aureus strains with different biofilm- forming abilities. Peer J. 2014; 2: e326.
- Mandal MD, Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed. 2011; 1: 154–60.
- Mahady G, Huang Y, Doyle BJ, Locklear TD. Natural products as antibacterial agents. Bioactive Natural Products (Part O. 2008; 35: 423–44.
- Mathews KA. Management of wounds using honey. Compendium on Continuing Education for Veterinarians Practicing. 2002; 24: 61.
- Manisha DM, Shyamapada M. Honey: its medicinal property and antibacterial activity. Asian Pacif Journal of Tropical Biomedicine. 2011; 154–60.
- Mohapatra DP, Thakur V, Brar SK. Antibacterial efficacy of raw and processed honey. Biotechnol Res Int. 2011; 2011.
- Molan P. Honey: antimicrobial actions and role in disease management. In: Ahmad I, F. Aqil 2009 Eds, editors. New Strategies Combating Bacterial Infection. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA. 2009; 229-253.
- Albaridi NA. Antibacterial Potency of Honey”. International Journal of Microbiology. 2019; 2019: 2464507.
- Nzeako BC, Hamdi J. Antimicrobial potential of honey on some microbial isolates. Sultan Qaboos University Journal of Scientific Research Medical Sciences. 2000; 2: 75-79.
- Escuredo O, Seijo MC. Authenticity of Honey: Characterization, Bioactivies and Sensorial properties. Eds., editor. Book: MDPI Publications. 2022; 11: 1301.
- Osata MS, Reddy SG, Graham DY. Osmotic effect of honey on growth and viability of Helicobacter pylori. Digestive Diseases and Sciences. 1999; 44: 462-464.
- Parri E, Santinami G, Domenici V. Front-Face Fluorescence of Honey of Different Botanic Origin: A Case Study from Tuscany (Italy. Appl Sci. 2020; 10: 1776.
- Paul IM, Beiler J, McMonagle A, Shaffer ML, Duda L, Cm B Jr. Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch Pediatr Adolesc Med. 2007; 161: 1140–6.
- Khalil D, El-Zayat SA, El-Sayed M. Phytochemical screening and antioxidant potential of Endophytic Fungi Isolated from Hibiscus sabdariffa. Journal of Applied Biotechnology Reports. 2020; 7: 116–24.
- Kwakman PH, Zaat SA. Antibacterial components of honey. IUBMB Life. 2012; 64: 48–55.
- Postmes T, Bogaard AE, Hazen M. Honey for wounds, ulcers, and skin graft preservation. Lancet. 1993; 341: 756–75.
- Sackett WG. Honey as carrier of intestinal diseases. Exp Station Colorado Agric College Bull. 1919; 252-266.
- Slover CM, Danziger LH, Adeniyi BA, Mahady GB. Use of natural products to combat multidrug-resistant bacteria. In: Ahmad I, F. Aqil 2009 Eds, editors. New Strategies Combating Bacterial Infection. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA. 2009; 127-135.
- Stephens JM, Schlothauer RC, Morris BD, Yang D, Fearnley L, Greenwood DR, et al. Phenolic compounds and methylglyoxal in some New Zealand manuka and kanuka honeys. Food chemistry. 2009; 120: 78–86.
- Subrahmanyam M, Hemmady A, Pawar SG. Antibacterial activity of honey on bacteria isolated from wounds. Annals of Burns and Fire Disasters. 2001; 14: 198–201.
- Taormina PJ, Niemira BA, Beuchat LR. Inhibitory activity of honey against foodborne pathogens as influenced by the presence of hydrogen peroxide and level of antioxidant power. International Journal of Food Microbiology. 2001; 69: 217-225.
- Vallianou N. Honey and its anti- inflammatory, anti-bacterial and anti-oxidant properties. General Med. 2014; 2.
- Vanketel BA. Feestnummer der Berichsten van de Nederlandische Maatschappij ter Bevordering der Pharmacie. 1892; 67 96.
- Organization WH. Traditional Medicine Strategy, World Health Organization (WHO. Geneva, Switzerland; 2014.
- Xing J, Guyer D, Ariana D, Lu R. Determining optimal wavebands using genetic algorithm for detection of internal insect infestation in tart cherry. Sens Instrum Food Qual Saf. 2008; 2: 161–7.
- Zainol MI, Yusoff KM, Yusof MYM. Antibacterial activity of selected Malaysian hphenoloney. BMC Complementary and Alternative Medicine. 2013; 13: 129.