
Research Article
J Dis Markers. 2023; 8(2): 1058.
Using the Gut Antigen HA86 as a Biomarker for Hyalomma Tick Bites, the Principal Vectors of the Crimean-Congo Hemorrhagic Fever Virus
Moufid Mhamadi1,2#*; Aminata Badji3#; Moundhir Mhamadi4; Alioune Gaye3; Idrissa Dieng3; El Hadji Ndiaye3; Mignane Ndiaye1; Babacar Faye2; Ousmane Faye1; Amadou Alpha Sall1; Gary Kobinger5; Hugues Fausther-Bovendo5; Mawlouth Diallo3; Oumar Faye1
1Department of Virology, Institut Pasteur de Dakar, Dakar 12900, Senegal
2Department of Parasitology, Université Cheikh Anta Diop de Dakar, Dakar 10700, Senegal
3Department of Medical Zoology, Institut Pasteur de Dakar, Dakar 12900, Senegal
4Institut Pasteur de Dakar, DIATROPIX, Dakar 12900, Senegal
5University of Texas Medical Branch, Galveston, TX 77555-0132, USA
*Corresponding author: Moufid Mhamadi Department of Virology, Institut Pasteur de Dakar, Dakar 12900, Senegal. Email: moufid.mhamadi@pasteur.sn
#These authors have equally contributed to this article.
Received: October 13, 2023 Accepted: November 22, 2023 Published: November 29, 2023
Abstract
Hyalomma ticks are the principal vectors of the Crimean-Congo hemorrhagic fever virus. Antibody responses and the viral genome, are usually used for virus surveys. These markers cannot assess the role of ticks in the burden of CCHFV. It has been documented that tick bites induce a host immunological response, and numerous antigens have been described. To note, the gut protein Bm86 of Boophilus micropulus induces such an immune response, and several orthologues have been identified. Furthermore, a cross-immunological response against orthologues was reported. Based on these findings, we hypothesized that the Bm86 of Hyalomma ticks could serve as a biomarker of exposure to Hyalomma tick bites.
For this purpose, 2432 ticks, 435 human and 480 animal sera, collected in Agnam (Northern Senegal) between February 2021 and April 2022 for arbovirus and viral hemorrhagic fever surveys were used.
We found that the development of HA86 IgG in livestock sheep is influenced by exposure to Hyalomma ticks. Additionally, the anti-HA86 antibodies did not persist for more than 2 months, and no cross-HA86 response wasn’t detected between Hyalomma and Rhipicephalus species. These results suggest that HA86, could be used as a biomarker for Hyalomma tick bites.
Keywords: Ticks; Hyalomma; Biomarker; HA86; Antibody
Context
Crimean-Congo Hemorrhagic Fever (CCHF) is the most prevalent tick-borne arbovirus in the world. Hard ticks (Ixodidae) of the Hyalomma genus are the main vectors of the disease [9]. It has been established that the Crimean-Congo Hemorrhagic Fever Virus (CCHFV) can be transmitted to the host by a competent tick carrying the CCHFV during a blood meal on a vertebrate host [9]. In humans, the infection could also occur after contact with infected tissues or fluids, such as during the slaughter of infected livestock or when caring for infected patients [17]. Several components are inoculated into the host during this blood meal, leading to a chain of reactions at the host level, which cause the production of markers of infection or exposure. Virological markers, such as the presence of viral genome or antibodies against the virus, are typically used to diagnose infection or exposure to CCHFV. Studies on the kinetics of this virus show that viral RNA can be detected in the blood by RT-PCR for up to 18 days after the onset of the first symptoms. IgM and IgG antibodies remain detectable for at least 4 to 6 months and 5 years, respectively [4]. These virological markers allow determination of the burden of CCHFV on the population's health. However, these markers cannot directly determine the impact of vector populations on the epidemiology of CCHFV. It is worth noting that few studies have documented exposure to tick bites, even though the immunological response to tick bites was described nearly a century ago [16].
For the record, many proteins in the gut of ticks have been shown to be immunogenic for vertebrate hosts [5-7,11]. It has been established that vertebrate hosts develop an immune response against these antigens after multiple infestations [12]. To date, the gut protein Bm86 from Boophilus micropulus ticks is the most well-documented. Its immunogenic role has been utilized to develop livestock vaccines against B. micropulus tick infestations [12]. Studies have shown that immunity induced by Bm86 protected cattle against other species of the same genus, such as B. appendiculus, B. annulatus, and B. decoloratus [5,15]. Similarly, a cross-immune response against ticks of the Hyalomma genus was documented with the Hd-86 ortholog of Bm-86 identified in Hyalomma scupense (syn. H. detritum) [8]. The high percentage of amino acid conservation (between 87 and 91%) between this Bm-86 ortholog of ticks could explain this cross-immune response [2,3]. Thus, we hypothesize that this cross-antibody response could be used as a biomarker for tick bite exposure. In this work, we propose to develop a serological test for the detection of antibody responses against a Bm-86 antigen of Hyalomma ticks, the main vectors of CCHFV.
Methodology
Hyalomma Bm86-HIS Orthologue
As no commercial Bm86 like protein of Hyalomma ticks was available, we aim to develop it. For this purpose, the Hyalomma Bm86 like protein, codons 1-646 of the Hyalomma anatolicum BM86-like gut protein (AAL36024.1) were synthesized and a Histidine tag (HIS) was added downstream. Then, HEK293T cells were cultured in 24-well plates and transfected using polyethyleneimine (PEI, Polysciences, France) with a 1:3 ratio of PEI to Bm86-HIS plasmid according to the manufacturer's instructions. Supernatants were collected 3 days after transfection and clarified by centrifugation at 8000g. The Bm86-HIS protein was then purified using nickel agarose resin (Ni-NTA) (Qiagen, Toronto, Canada) according to the manufacturer's instructions. The purity of the Bm86-HIS protein obtained was analyzed by SDS-PAGE. The generated antigen was labeled HA86.
Samples Used for the Study
The samples used from our study were collected in the One Health site established in the Agnam area as previously described [14]. The Agnam area is an arid zone located in the region of Matam (15°06′ 18″ N and 13°38′ 30″ W) in the northeast of Senegal. CCHFV circulates in this area on human-animal and Hyalomma impeltatum constitutes the dominant tick species in Agnam. Other species of this genus have been listed: H. marginatum rufipes and H. truncatum [14]. For the development of the test and for the evaluation of populations exposition to Hyalomma tick bites, we selected human and animal sera as well as ticks collected between February 2021 to April 2022 in Agnam areas. A total of 413 human samples, 480 samples from the 32-sentinel sheep, and 2432 ticks were selected for this study.
Detection of Anti-HA86 Antibodies
For the detection of Immunoglobulin G (IgG) antibodies against Hyalomma tick antigen 86 (HA86), an indirect ELISA was used. Briefly, 96-well plates were coated with 500 ng/mL of the HA86 protein diluted in Phosphate Buffer Saline 1x (PBS). These plates were then sealed and incubated overnight at 4 °C. Then plates were washed 3 times by adding 300μL of washing buffer (PBS 1x + Tween 20 at 0.05%) and the antigenic sites were blocked using a blocking buffer (PBS 1x + Tween 20 at 0.05% + 5% skimmed milk). After 1 hour of incubation at 37°C, plates were washed 3 times then we added the 1/100 diluted sera and we incubated them again at 37°C for 1 hour. After washing, a species-specific antibody (rabbit anti-sheep IgG (Biorad) or a goat anti-human IgG (KPL)) was conjugated with horseradish peroxidase then the plates were incubated at 37°C for 1 hour. The plates were then washed and 3, 3 ′, 5, 5′-tétraméthylbenzidine (TMB SIGMA Aldrich, USA) was added. The reaction was stopped with sulfuric acid after 5 minutes. Optical Densities were read with the 450/620 filters and the cut-off was determined using the finite mixture model.
Data Analysis
The data generated were entered in Excel and the statistical analyzes were performed on the R software using the R studio interface. Using the finite mixture model on Optical Densities (OD) as previously described [13], the cut-off threshold was determined. Subsequently, the difference in antibody levels and HA86 IgG carriage between 2 groups was analyzed by logistic regression. Linear regression was used in order to determine the influence of number of ticks on antibody levels. The correlation of HA86 specific IgG between 2 independent groups was calculated with a Pearson test. The development of HA86 immunity during the time of exposition to ticks bites was represented by the Kaplan Maier curve and compared by Log-rank (Mantel-Cox) test. The effect of time exposition to ticks bites on the development of HA86 immunity was tested by Khi-square test. The significance level was set at 0.05% (p<0.05).
Results
Ticks Distribution
Out of the 2432 ticks selected, seven species were identified between February 2021 and April 2022: H. impeltatum (78.86%), H. marginatum rufipes (0.20%), H. truncatum (1.02%), R. guilhoni (12.33%), R. mushamae (3.45%), R. sulcatus (2.17%), and R. evertsi evertsi (1.93%). H. impeltatum was collected every month except for September and October 2021. Among the species of the Rhipicephalus genus, Rhipicephalus guilhoni was identified throughout the entire 14-month survey. The other species were rarely collected (Figure 1).
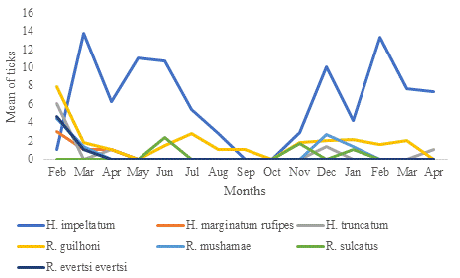
Figure 1: Temporal distribution of the ticks collected on sheep in Idite.
HA86 in Humans and Animals
Out of the 453 sera tested for the presence of antibodies directed against the HA86 tick antigen, none tested positive. However, analysis of blood samples taken from 32 sheep revealed that 16 sheep carried anti-HA86 antibodies (50.00%). Our analysis showed that HA86 IgG was detected only in animals exposed to H. impeltatum and H. truncatum, but the number of ticks had no influence on seropositivity for H. impeltatum (p=0.813) and H. truncatum (p=0.172) (Figure 2).
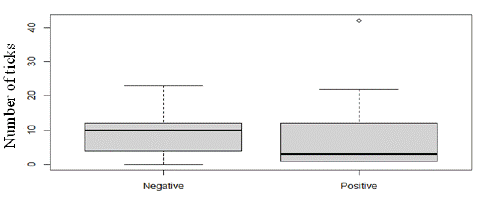
Figure 2: Influence of number of ticks in anti-HA86 antibody levels.
Similarly, the number of ticks did not induce any modification in HA86 antibody levels for H. impeltatum (p=0.895) and H. truncatum (p=0.317).
We noticed that exposure to H. impeltatum tick bites significantly changed the levels of HA86 IgG antibody (p<0.0001). On the other hand, exposure to H. truncatum tick bites did not induce any variation in HA86 IgG levels, as the p-value was not significant (p=0.442) (Figure 3).
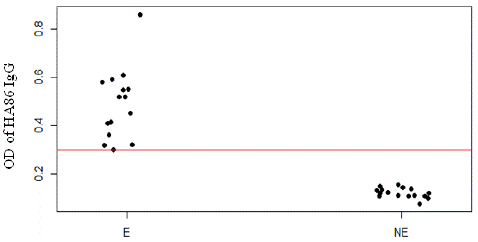
Figure 3: Anti-HA86 antibody levels between sheep infested (E) by Hyalomma ticks and non-infested sheep (NE). The red line represents the cut-off value of anti-HA86 IgG (0.299).
The HA86 IgG cross-reactivity analysis between HA86-positive animals exposed to H. impeltatum and H. truncatum tick bites showed a significant strong correlation in the immune response (r=-0.98, CI [-0.99, -0.37], p=0.017) (Figure 4).
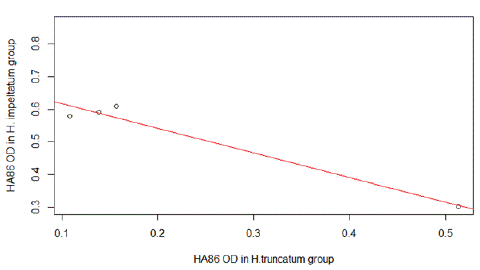
Figure 4: Cross anti-HA86 immunity detected between the group infested by H.truncatum and the group infested H. impeltatum.
Between February 2021 and April 2022, HA86 IgG increased proportionally with the number of exposures to Hyalomma ticks. More than 60% of HA86 IgG was detected at the end of the study in February, March, and April 2022 (Figure 5).
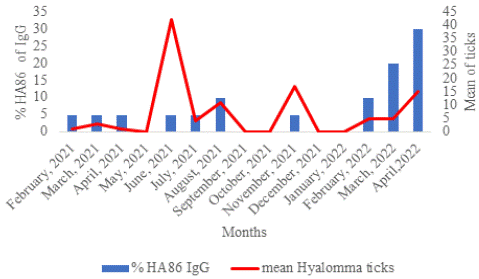
Figure 5: Temporal distribution of the anti-HA86 and Hyalomma ticks.
The number of exposures to H. impeltatum tick bites seems to have an influence on HA86 IgG carriage in sheep, as statistical significance was observed (p<0.0001). We observed that the HA86 immune response did not persist beyond 2 months, and HA86 IgG did not confer immunity against tick infestation. Kaplan-Meier analysis revealed that consecutive H. impeltatum infestation during the 416 days of sample collection induced the development of HA86 IgG (p<0.0001).
Discussion
Ticks of the genus Hyalomma are the primary vectors of the Crimean-Congo hemorrhagic fever virus [10]. Developing a biomarker to assess exposure to tick bites from this genus would provide insights into the impact of its vectors on population health. Therefore, we aimed to develop a test for detecting the intestinal protein HA86 specific to Hyalomma ticks.
Our data revealed that 80.02% of the ticks collected during this study were identified as Hyalomma ticks. This finding suggests that Hyalomma ticks are the major vectors of CCHFV in this region. This result is supported by a recent study where more than half of the ticks collected across eight Senegalese regions were identified as Hyalomma ticks [13].
The results of our study showed a prevalence of 0% in humans tested between June 2021 and June 2022. This prevalence indicates that the transmission of CCHF to Agnam is unlikely to be primarily due to tick bites. This hypothesis is further supported by the fact that this prevalence is lower than the reported prevalence of 10.44% for anti-CCHFV IgM/IgG antibodies in humans at Agnam [14]. Therefore, the transmission of CCHF to humans in Agnam could be attributed to direct contact with tissues from infected hosts [17].
Regarding livestock, the analysis showed that 16 out of the 32 selected sheep developed anti-HA86 IgG antibodies during the study period. The prevalence of 50.00% found in our study is comparable to the prevalence of 38.24% of anti-CCHFV IgG antibodies reported in the same ruminants at Agnam in 2021 [14]. Furthermore, our findings indicate that the majority of anti-HA86 IgG antibodies were detected in February, March, and April 2022, which aligns with a previous study in Agnam showing that the peak of CCHFV transmission occurs during the same period in sentinel sheep [14]. These results suggest that in Agnam, CCHFV is primarily transmitted to sheep through tick bites from the Hyalomma genus.
We found that the anti-HA86 response was only detected in animals exposed to H. impeltatum and H. truncatum. These findings suggest that the HA86 IgG response is specific to the Hyalomma tick group. This hypothesis is confirmed by the fact that a cross-immune HA86 response is detected between these two groups. Previous studies have also reported a cross-immune response for Bm86 homolog in Hyalomma ticks [8].
We observed that no HA86 IgG antibodies were detected in animals infested by H. marginatum rufipes. This could be attributed to the fact that these Hyalomma species were rarely collected during our study. In fact, our results indicate that multiple infestations by H. impeltatum induce an HA86 IgG response, and previous studies have demonstrated that multiple blood meals are necessary for the development of an immune response against these hidden antigens [12].
Furthermore, we noticed that anti-HA86 antibodies were detected even in blood samples taken 14 or 30 days apart, indicating that the HA86 IgG response does not provide efficient immunity against Hyalomma tick infestation. These findings suggest that to use HA86 antigen as a vaccine against Hyalomma tick infestation, it would need to be combined with a host immune booster. Overall, these findings indicate that anti-HA86 IgG antibodies could serve as a biomarker for exposure to Hyalomma tick bites. In this study, anti-HA86 antibodies allowed us to demonstrate that the transmission of CCHFV in sheep is mainly ensured by Hyalomma ticks, while human transmission occurs primarily through direct contact. Due to their specificity to Hyalomma ticks, anti-HA86 IgG antibodies represent a valuable biomarker for tick bite exposure from the Hyalomma genus.
To determine the kinetics of anti-HA86 antibodies, laboratory infestation experiments could be conducted using different species of Hyalomma as well as other tick species.
Author Statements
Author Contributions
M.M. (Moufid Mhamadi), O.F (Oumar Faye) and M.D design the study. M.M. (Moufid Mhamadi), M.M. (Moundhir Mhamadi), A.B. (Aminata Badji), M.N. (Mignane Ndiaye) collect the samples and perform the analysis; M.M. (Moufid Mhamadi) writhed the original draft.; M.M. (Moufid Mhamadi), A.B. (Aminata Badji), I.D., O.F. (Oumar Faye), M.D., A.G., E.H.N., B.F., H.F.-B., O.F. (Ousmane Faye)., A.A.S. and G.K review and editing the manuscript. All authors have read and agreed to the submitted version of the manuscript.
Funding
This research was funded by 2 grants from the International Development Research Center (grant 109075-001) and the Canadian Department of Global Affairs (grant BIO-2019-005).
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the National Ethical Committee for Health and Research in Senegal with ethics approval number 00000806 MSAS/DPRS/DR for the studies involving humans and animals. We confirm that this study complies with all regulations and we confirm that informed consent was obtained from the entire patient involved in this study.
Data Availability Statement
The data used in this study are available on request from the corresponding author.
Acknowledgments
We acknowledge our colleagues of IPD for their tremendous support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Badji A, Ndiaye M, Gaye A, Dieng I, Ndiaye EH, Dolgova AS, et al. Detection of Crimean–Congo haemorrhagic fever virus from livestock ticks in northern, central and Southern Senegal in 2021. Trop Med Infect Dis. 2023; 8: 317.
- Ben Said M, Galai Y, Canales M, Nijhof AM, Mhadhbi M, Jedidi M, et al. Hd86, the Bm86 tick protein ortholog in Hyalomma scupense (syn. H. detritum): expression in pichia pastoris and analysis of nucleotides and amino acids sequences variations prior to vaccination trials. Vet Parasitol. 2012a; 183: 215-23.
- Ben Said M, Galai Y, Mhadhbi M, Jedidi M, de la Fuente J, Darghouth MA. Molecular characterization of Bm86 gene orthologs from Hyalomma excavatum, Hyalomma dromedarii and Hyalomma marginatum marginatum and comparison with a vaccine candidate from Hyalomma scupense. Vet Parasitol. 2012b; 190: 230-40.
- Burt FJ. Laboratory diagnosis of Crimean–Congo hemorrhagic fever virus infections. Future Virol. 2011; 6: 831-41.
- Canales M, Almazán C, Naranjo V, Jongejan F, de la Fuente J. Vaccination with recombinant Boophilus annulatus Bm86 ortholog protein, Ba86, protects cattle against B. annulatus and B. microplus infestations. BMC Biotechnol. 2009; 9: 29.
- de la Fuente J, Rodríguez M, Montero C, Redondo M, García-García JC, Méndez L, et al. Vaccination against ticks (Boophilus spp.): the experience with the Bm86-based vaccine Gavac. Genet Anal. 1999; 15: 143-8.
- Ebrahimi SM, Paykari H, Memarnejadian A. Molecular characterization of HAO3, the homologue of the Bm86 tick vaccine antigen, from the Iranian isolate of Hyalomma anatolicum anatolicum. Exp Parasitol. 2013; 135: 726-34.
- Galaï Y, Canales M, Ben Saïd M, Gharbi M, Mhadhbi M, Jedidi M, et al. Efficacy of Hyalomma scupense (Hd86) antigen against Hyalomma excavatum and H. scupense tick infestations in cattle. Vaccine. 2012; 30: 7084-9.
- Gargili A, Estrada-Peña A, Spengler JR, Lukashev A, Nuttall PA, Bente DA. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: a review of published field and laboratory studies. Antiviral Res. 2017; 144: 93-119.
- Hoogstraal H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol. 1979; 15: 307-417.
- Kamau LM, Skilton RA, Githaka N, Kiara H, Kabiru E, Shah T, et al. Extensive polymorphism of Ra86 genes in field populations of Rhipicephalus appendiculatus from Kenya. Ticks Tick-Borne Dis. 2016; 7: 772-81.
- Kemp DH, Pearson RD, Gough JM, Willadsen P. Vaccination againstBoophilus microplus: localization of antigens on tick gut cells and their interaction with the host immune system. Exp Appl Acarol. 1989; 7: 43-58.
- Mhamadi M, Badji A, Barry MA, Ndiaye EH, Gaye A, Ndiaye M, et al. Human and livestock surveillance revealed the circulation of rift valley fever virus in agnam, Northern Senegal, 2021. Trop Med Infect Dis. 2023; 8: 87.
- Mhamadi M, Badji A, Dieng I, Gaye A, Ndiaye EH, Ndiaye M, et al. Crimean-Congo hemorrhagic fever virus survey in humans, ticks, and livestock in agnam (Northeastern Senegal) from February 2021 to March 2022. Trop Med Infect Dis. 2022; 7: 324.
- Odongo D, Kamau L, Skilton R, Mwaura S, Nitsch C, Musoke A, et al. Vaccination of cattle with TickGARD induces cross-reactive antibodies binding to conserved linear peptides of Bm86 homologues in Boophilus decoloratus. Vaccine. 2007; 25: 1287-96.
- Trager W. Acquired immunity to ticks. J Parasitol. 1939; 25: 57-81.
- Whitehouse CA. Crimean-Congo hemorrhagic fever. Antiviral Res. 2004; 64: 145-60.