
Research Article
J Dis Markers. 2023; 8(2): 1057.
Alternation in the Abundance and Phosphorylation Status of Aquaporin-2 in the Serum Exosome of Patients with Chronic Kidney Disease
Yao-Te Chung1,5; Chiung-Hu Kuo2; Ru-Yi Tsai3; Jin-Wun Chen4; Chin Li5; Wen-Yi Li6*
1Department of First Common Laboratory, National Taiwan University Hospital, Yunlin Branch, Yun-Lin, Taiwan
2Department of Laboratory Medicine, National Taiwan University Hospital Chutung Branch, Hsinchu, Taiwan
3Department of Cardiovascular Center, National Taiwan University Hospital Yunlin Branch, Yun-Lin, Taiwan
4Department of First Common Laboratory, National Taiwan University Hospital Yunlin Branch, Yun-Lin, Taiwan
5Department of Biomedical Sciences, National Chung-Cheng University, Chia-Yi, Taiwan
6Department of Internal Medicine, National Taiwan University Hospital Yunlin Branch, Yun-Lin, Taiwan
*Corresponding author: Wen-Yi Li Department of Internal Medicine, National Taiwan University Yunlin Branch, 579, Sec 2, Yunlin Road, Douliu City, Yunlin 640, Taiwan Tel: 886-5-5323911; Fax: 886-5-5373257 Email: Y00905@ms1.ylh.gov.tw
Received: August 01, 2023 Accepted: August 28, 2023 Published: September 04, 2023
Abstract
Background: Exosomes, which are vesicles ranging from 40 to 160 nm in size, are released from cellular membranes and serve as a means of cell-to-cell communication by delivering their contents, such as microRNA and protein, to recipient cells. These extracellular vesicles are present in human blood and urine and their composition can be altered in specific pathogenic conditions. Aquaporin-2 (AQP2) is a critical aquaporin involved in water homeostasis and has been identified as an important biomarker in chronic kidney disease. However, it is unclear whether AQP2 is transported via exosomes.
Method: This study included 16 participants with chronic kidney disease and 11 healthy volunteers. Serum exosomes were isolated through polymer precipitation and imaged using transmission electron microscopy. The diameter of purified serum exosomes was measured via flow cytometry, and their identity was further confirmed by identifying exosome markers through immunoblotting. Additionally, the participants’ hepatic B and C virus infection status was determined using chemiluminescent microparticle immunoassay.
Results: The precipitation method was utilized to isolate serum exosomes, and successful purification was confirmed through transmission electron microscopy imaging. Size estimation was conducted via flow cytometry, and the presence of exosome markers CD63, TSG101, CD9, and CD81 was confirmed through immunoblotting. Furthermore, the presence of AQP2 was detected in serum exosomes, and its abundance was found to significantly decrease in patients with chronic kidney disease. However, despite the decreased abundance, the percentage of phosphorylated AQP2 at serine 256 was significantly increased.
Conclusion: Our findings suggest that changes in the abundance and phosphorylation status of AQP2 on serum exosomes are associated with the development of chronic kidney disease, and could potentially serve as a signal for exosome transport.
Keywords: CKD; AQP2; Exosme
Lay Summary
Exosomes, small vesicles involved in cell communication, carry molecules between cells. This study examined Aquaporin-2 (AQP2), a protein important in water balance, and its presence in exosomes, specifically in Chronic Kidney Disease (CKD). Blood samples were collected from CKD patients and healthy individuals, and exosomes were isolated and characterized. AQP2 was found in exosomes, and its levels were significantly lower in CKD patients compared to healthy individuals. Interestingly, phosphorylation of AQP2 at a specific site was increased in CKD patients. These findings suggest that changes in AQP2 abundance and phosphorylation in exosomes may be linked to CKD development. Understanding exosome-associated changes in AQP2 could offer insights into CKD progression and potentially lead to new diagnostic markers or treatment strategies. Further research is needed to explore the underlying mechanisms and clinical implications of these findings.
Introduction
Exosomes are a type of extracellular vesicle that range in size from 40 to 160 nm in diameter, depending on the cell type they originate from. Monocytes and macrophages, for example, can produce exosomes that are larger, with an average size of up to 200 nm [1]. Endothelial cells, on the other hand, tend to produce exosomes that are around 150 nm in diameter [2]. These vesicles are capable of carrying various types of biomolecules, including DNA, mRNA, microRNA, and proteins, and can act as vehicles to transport cargo to target cells in a paracrine or endocrine manner [3,4]. Additionally, exosomes have been found to play a role in viral infections [5,6], making them a versatile and multi-functional means of intercellular communication.
Exosomes often exhibit disease-specific changes, making them potential diagnostic or prognostic biomarkers. In the context of kidney-related pathologies, various biomarkers have been investigated. Studies have demonstrated significant changes in exosomal microRNAs during the progression of diabetic nephropathy [7], while exosomal AQP2 has been evaluated as a potential biomarker for renal dysfunction [8]. In addition, general circulation microparticles have been utilized to monitor vascular dysfunction in end-stage renal failure [9]. However, whether there are discernable alterations in circulating exosomes of individuals with chronic kidney disease remains unclear.
Ultracentrifugation and precipitation are the two primary methods for isolating exosomes from liquid biopsies. Precipitation, which involves the use of high-molecular-weight polymers such as PEG, can be performed without special equipment and is a popular alternative to ultracentrifugation [10]. While ultracentrifugation yields a lower quantity of exosomes, it produces a much purer sample. However, due to the requirement for an ultra-high-speed centrifuge, this method is less commonly used for routine exosome isolation. Confirmation of isolated exosomes can be achieved through various experimental techniques. For instance, flow cytometry analysis can be employed to determine particle diameter. In addition, immunoblotting can be used to detect known exosome transmembrane protein markers, such as CD63, CD9, and CD81, as well as the cargo protein TSG101, which serve as positive identification for exosomes in purified samples [11-13].
Aquaporins are integral membrane proteins that regulate water homeostasis by serving as water channels. AQP2, a member of the aquaporin family, is involved in endocytosis and exocytosis and is also incorporated into exosomes that are subsequently released into the blood or urine. The activity of AQP2 is modulated by phosphorylation and ubiquitination. Studies have demonstrated that serine-256 phosphorylation of AQP2 is associated with trafficking between intracellular spaces via vesicles [14], while threonine-269 and serine-261 phosphorylation is involved in secretion [15]. However, the relationship between AQP2 phosphorylation and exosome secretion is not well understood. In this study, we isolated and analyzed exosomes from serum samples collected from healthy volunteers and patients with chronic kidney disease. Our findings revealed that the concentration of exosomes was significantly lower in patients with chronic kidney disease than in healthy individuals. Despite the lower exosome concentration, the ratio of serine-256 phosphorylated AQP2 to unphosphorylated AQP2 was considerably higher in patients compared to healthy volunteers. Our study highlights the potential of using the concentration of serum exosomes and phosphorylated AQP2 as a signal for evaluating chronic kidney disease.
Materials and Methods
Study Design
The flow chart depicts the essential components of the study design (Figure 1). In this study, serum exosomes were isolated using polymer precipitation and visualized using transmission electron microscopy. The size of purified serum exosomes was determined using flow cytometry, while their identity was confirmed through the identification of exosome markers via immunoblotting.
Figure 1: The flow chart depicts the essential components of the study design.
Study Cohort
The study was carried out at National Taiwan University Hospital Yunlin Branch in compliance with the principles of the Declaration of Helsinki. The collection and utilization of clinical specimens were conducted in adherence to ethical guidelines, and all participants were provided with detailed information about the study's objectives and provided written informed consent. The Research Ethics Committee of National Taiwan University Hospital reviewed and monitored the study, with approval numbers 201717001RIND. The study recruited a total of 16 patients with chronic kidney disease and 11 healthy volunteers from the National Taiwan University Hospital Yunlin Branch between 2017 and 2018. Table 1 presents an overview of the clinicopathological characteristics of the participants. Inclusion criteria for patients required a first-time diagnosis that necessitated dialysis therapy, such as an eGFR < 15 (mL/min/1.73 m2). Healthy volunteers were eligible to participate if they did not have chronic kidney disease.
N
CKD
p value
n
11
16
age
38±9
69±9
Na(mmol/L)
138.9±1.0
135.3±5.4
0.0192
K(mmol/L)
3.9±0.3
4.3±0.7
0.036
Ca(mmol/L)
2.32±0.06
2.20±0.21
0.044
P(mg/dL)
3.3±0.8
5.5±1.7
0.0001
Creatinine(mg/dL)
0.7±0.2
12.6±5.0
<0.0001
eGFR
(mL/min/1.73 m2)102.8±15.1
5.4±6.4
<0.0001
Table 1: This study evaluated the clinopathogenic characteristics of patients with Chronic Kidney Disease (CKD) and healthy volunteers (N), including age, gender, kidney function.
Specimen Collection and Kidney Function Evaluation
Each study participant provided a 5-mL whole blood specimen, which was collected in a BD Vacutainer SST Blood Collection Tube. The collected blood specimens were centrifuged at 1600×g and 4°C. After centrifugation, the serum was collected and stored at -70°C for further analysis. The subjects' kidney function was evaluated, and their estimated glomerular filtration rate (eGFR) was calculated using the modification of diet in renal disease method.
Exosome Isolation
To isolate serum exosomes, we used the polymer precipitation method [16]. The serum was first centrifuged at 3000×g to remove any residue, and the resulting supernatant was then filtered through a 0.22-μm membrane. The filtrate was then mixed with Polyethylene Glycol (PEG) to obtain a final concentration of 8% PEG. The mixture was then incubated at 4°C for 30 minutes, and the serum exosomes were subsequently precipitated by centrifugation at 1500×g. The resulting pellet was suspended in a phosphate-buffered solution, resulting in a final preparation of purified exosomes.
Transmission Electron Microscope Imaging
To visualize the purified exosomes, Transmission Electron Microscopy (TEM) was employed following negative staining [17]. The procedure involved several steps. Firstly, a hydrophilic formvar carbon film was deposited onto a copper grid through glow discharge. Subsequently, 4 μL of purified serum exosomes were applied to the film and allowed to incubate for 1 minute. Excess solution was carefully removed using filter paper, and the film was rinsed with 4 μL of filtered deionized water. The exosomes were then stained with 2% uranyl acetate at pH 7 for 1 minute. After removing the excess staining solution, the film was air-dried. Finally, the stained serum exosomes were visualized using TEM at a magnification of 60,000×. The exosomes appeared as particles ranging from approximately 40 to 160 nm in size.
Flow Cytometry Analysis
To determine the diameter of serum exosomes, flow cytometry was conducted using the Beckman Coulter CytoFLEX instrument. A nanobead calibration kit (Bangs Laboratories, Inc. Catalog number 834, Lot number 12771) and a submicron calibration kit (Bangs Laboratories, Inc. Catalog number 832, Lot number 10068) were employed for calibration purposes. The calibration beads provided in these kits had diameters of 0.05 μm, 0.1 μm, 0.2 μm, 0.5 μm, and 0.8 μm. The calibration process aimed to collect 50,000 events specifically for the 0.1 μm beads. This ensured a sufficient number of recorded instances of the 0.1 μm beads passing through the flow cell, facilitating accurate calibration. Following the completion of the calibration step, the cytometer was flushed with PBS (phosphate-buffered saline) before analyzing the actual exosome samples. Each sample was diluted with PBS and subsequently analyzed using flow cytometry. In this study, exosomes with a diameter of 0.2 μm or less were considered. This included both the population of exosomes smaller than 0.1 μm and those with diameters ranging from 0.1 μm to 0.2 μm. Additionally, PBS was included as a blank in each sample. This allowed for the assessment of background noise or non-specific binding during the analysis process.
Immunoblotting
To extract serum exosome proteins, luciferase cell culture lysis reagent (Promega) was utilized during the lysate preparation process, supplemented with proteinase and phosphatase inhibitors. Protein concentrations were determined using the Bradford method (Sigma), with beta-globin serving as the standard. Equal amounts of protein samples were separated via 12% SDS-polyacrylamide gel electrophoresis and subsequently transferred to a PVDF membrane for protein detection. Immunoblotting was performed using the following primary antibodies: anti-CD63 (System Biosciences, Cat. Exoab-CD63A-1), anti-CD9 (System Biosciences, Cat. Exoab-CD9A-1), anti-CD81 (Abcam, EPR4244), anti-Alix (System Biosciences, Cat. Exoab-Alix-1), anti-TSG101 (System Biosciences, Cat. Exoab-TSG101-1), anti-AQP2 (St John’s Laboratory, Cat. STJ91655), and anti-phospho-AQP2 (S256) (St John’s Laboratory, Cat. STJ91138). The secondary antibody employed was anti-rabbit (System Biosciences, Cat. Exoab-HRP), and Immobilon western chemiluminescent HRP substrate (Millipore, Cat. WBKLS0500) was used for protein detection. The blotting results were captured using Fuji medical X-ray film (FUJI, Cat. 4741019291) and analyzed with Fusion software (Vilber). The film quantification area was used to indicate protein levels in each lane.
Hepatitis Virus Analysis
To detect hepatitis B and C viruses, a Chemiluminescent Microparticle Immunoassay (CMIA) method was employed. Exosomes were diluted with deionized water to a final volume of 500 μL, and 5 μL of the diluted exosomes were used for the assay. The CMIA assay was conducted at the Department of Laboratory Medicine in the National Taiwan University Hospital Zhudong Branch. In the context of the study, the symbol "+" was used to indicate a positive result, which means the signal-to-cutoff ratio (S/CO) is greater than or equal to 1.00. Conversely, the symbol "-" was used to indicate a negative result, which means the S/CO is less than 1.00 in the context of hepatitis diagnosis.
Statistical Analysis
The statistical difference in means between the healthy subject control and patient groups was compared using a two-sample t-test assuming unequal variances, also known as the Welch's t-test. A p-value less than 0.05 was considered statistically significant.
Results
Exosomes Isolation
In this study, we isolated serum exosomes using a polymer precipitation method and characterized their morphology and identity. Transmission Electron Microscopy (TEM) was used to visualize the exosomes, which exhibited a round morphology and a unimodal size distribution of 40-160 nm (Figure 2A). To further confirm the identity of the isolated exosomes, we performed flow cytometry analysis to determine the particle diameter. Our results showed that 99% of the serum exosomes had diameters less than 0.2 μm, with the major population being approximately 0.1 μm (Figure 2B). These characteristics were consistent with the typical size range and morphology of exosomes.
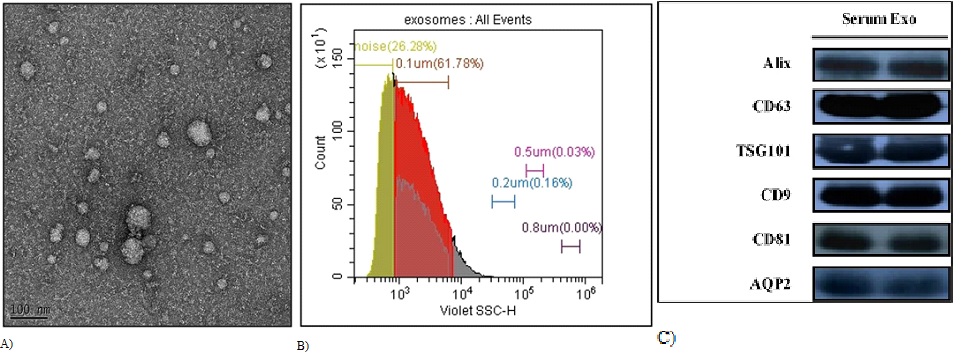
Figure 2: Our study confirms the identification of serum exosomes through multiple experimental techniques, including transmission electron microscopy, flow cytometry, and immunoblotting. (A) The purified exosomes were imaged at 60,000-fold magnification using transmission electron microscopy after being negatively stained. (B) Flow cytometry was used to assess the size of serum exosomes, with calibration bead diameters of 0.05 μm, 0.1 μm, 0.2 μm, 0.5 μm, and 0.8 μm. (C) In our immunoblotting analysis of common exosome markers, equal concentrations of total protein were loaded in each lane.
To further support the identification of our isolated exosomes, we performed biochemical characterization using immunoblotting analysis. Our results showed the presence of common exosome markers such as CD63, TSG101, CD9, and CD81 (Figure 2C). These findings confirm that our isolated samples are exosomes and validate the efficacy of the precipitation method used in this study. In conclusion, our study demonstrates successful isolation of serum exosomes using polymer precipitation and provides evidence for their identity through morphological and biochemical characterization.
Serum Exosomes in CKD
Several biomarkers have been evaluated for kidney-associated pathogenic conditions, and creatinine is a general biomarker for chronic kidney disease (Table 1). The study included subjects in stage 5 kidney failure with chronic kidney disease. We used flow cytometry to calibrate the subject's serum exosomes, and found lower levels of exosomes in patients with chronic kidney disease (Figure 3A). This finding provides an opportunity for diagnosing chronic kidney disease.
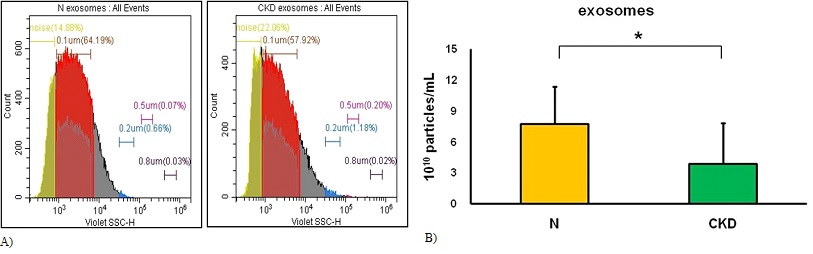
Figure 3: The study can perform serum exosome calibration using flow cytometry. (A) The endpoint of the calibration process was to achieve equal detection times between N (representing healthy volunteers) and CKD (representing patients with chronic kidney disease. (B) The cut-off point for the calibration process was determined to be less than 0.2-μm beads. *The results of the analysis showed a statistically significant difference between N (representing healthy volunteers, n=11) and CKD (representing patients with chronic kidney disease, n=16).
We evaluated exosomes with a diameter below 0.2 μm, and they found that healthy volunteers had 7.7±3.7×1010 particles/mL (n=11) while patients with chronic kidney disease had 3.8±4.0×1010 particles/mL (n=16) (Figure 3B). This difference could be used as a parameter for evaluating chronic kidney disease.
However, the association between exosomes and chronic kidney disease is poorly understood. To further investigate this association, we classified serum exosomes into two groups based on diameter: those with a diameter less than 0.1 μm and those with a diameter between 0.1 and 0.2 μm (Table 2). They found a significant alteration in the latter subpopulation, but not the former, indicating that alterations in patients with chronic kidney disease occur in the subpopulation of serum exosomes with a diameter between 0.1 and 0.2 μm.
<0.1 μm
(1010 particles/mL)0.1 μm~0.2 μm
(1010 particles/mL)Total
(1010 particles/mL)N
2.7±1.2
4.9±2.9
7.7±3.7
CKD
2.2±2.4
1.7±1.6
3.8±4.0
p value
0.441
0.004
0.016
*The study included a total 27 participants, with n=11 healthy volunteers and n=16 patients with Chronic Kidney Disease (CKD). The healthy volunteers were included as a control group for comparison with the CKD patients, who were in stage 5 kidney failure. PBS (phosphate-buffered saline) is a blank (or a blank solution) commonly used in flow cytometry calculations.
Table 2: The study classified the subpopulation of serum exosomes from the subjects based on diameter, with one group having a diameter less than 0.1 μm and the other group having a diameter between 0.1 and 0.2 μm.
Hepatic B and C in Serum Exosomes
Exosomes have been found to play a role in viral infections, including those caused by hepatic viruses. In patients with chronic kidney disease, hepatic viruses are frequently observed, and the detection of core antigen is commonly used for the diagnosis of hepatic viral infections [19]. Additionally, exosomes are known to have the function of antigen presentation [20]. In this study, the Chemiluminescent Microparticle Immunoassay (CMIA) was used to detect hepatic viruses, and 3.7% of subjects were found to have hepatitis B virus (HBV) and 14.8% were found to have Hepatitis C Virus (HCV) in their serum exosomes (Table 3).
Sample numbers
HBV
HCV
+
-
+
-
N
11
1(9.1%)
10
0(0%)
11
CKD
16
0(0%)
16
4(25%)
12
total
27
1(3.7%)
26
4(14.8%)
23
*In the context of the study, the symbol "+" indicates that the signal-to-cutoff ratio (S/CO) is greater than or equal to 1.00, while "-" indicates that the S/CO is less than 1.00.
Table 3: Hepatic viruses were detected in the serum exosomes of the study subjects. Specifically, 3.7% of the subjects had Hepatitis B Virus (HBV) and 14.8% had Hepatitis C Virus (HCV) detected in their serum exosomes.
To confirm these findings, the medical records of the subjects were checked for positive reactions that were consistent with the positive reactions observed in serum exosomes. Subjects who tested positive for hepatitis B or C virus were excluded from the study. The calibration of serum exosomes showed no significant differences between subjects with and without hepatitis virus infection (Figure 4 & Table 4). Therefore, the presence of hepatitis infection did not interfere with serum exosome calibration in this study.
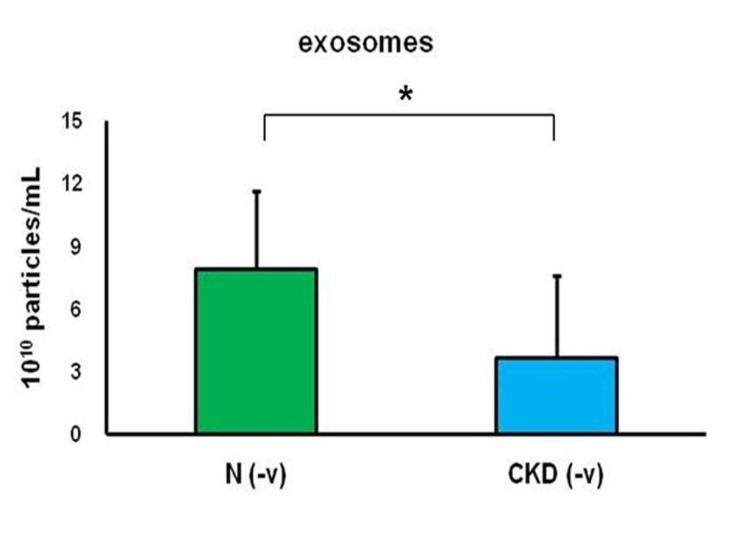
Figure 4: The calibration of serum exosomes was performed using flow cytometry, with a cutoff point of less than 0.2-μm beads. The groups N (-v) and CKD (-v) represent healthy volunteers (n=10) and patients with chronic kidney disease (n=12), respectively, who did not have hepatitis virus infection. The asterisk (*) indicates a statistically significant difference between the N (-v) and CKD (-v) groups
<0.1 μm
(1010 particles/mL)0.1 μm~0.2 μm
(1010 particles/mL)Total
(1010 particles/mL)N(-v)
2.8±1.3
5.1±3.0
7.9±3.8
CKD(-v)
2.0±2.5
1.6±1.5
3.7±3.9
p value
0.372
0.006
0.019
*The groups N(-v) (n=10) and CKD(-v) (n=12) represent healthy volunteers and patients with chronic kidney disease, respectively, who did not have hepatitis B or C virus infection.
Table 4: The subpopulations were classified based on the serum exosomes of subjects who did not have hepatitis B or C virus infection.
AQP2 on Serum Exosomes
Based on the results of immunoblotting analysis, it was confirmed that AQP2 is incorporated into serum exosomes and released into the blood or urine. Moreover, it was found that phosphorylation of AQP2 at serine 256 is an important signal in AQP2 trafficking during exosome transport. The study found that chronic kidney disease patients had lower exosome marker and AQP2 expression compared to healthy individuals (Figure 5A, B). Additionally, the abundance of AQP2 on exosomes was significantly decreased, but there was an increase in phosphorylation of serine 256 on exosome-incorporated AQP2 (Figure 4C). These findings suggest that the alteration of abundance and the relative phosphorylation status of AQP2 on serum exosomes may be correlated to the development of chronic kidney disease.
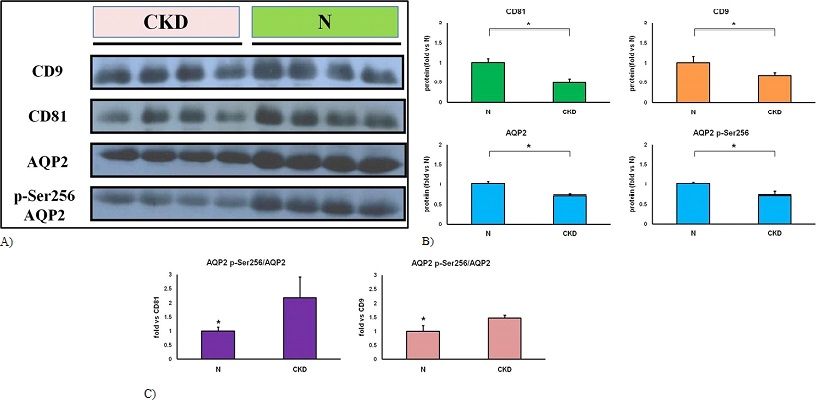
Figure 5: Detection of AQP2 using immunoblotting in serum exosomes. (A) Equal protein concentrations loaded in each lane. (B) Immunoblotting analysis was performed using Fusion software to analyze protein alterations in exosome markers and AQP2. The ratio of protein alteration was calculated by comparing CKD samples to N samples. Specifically, the ratio of AQP2 phosphorylated at serine 256 (AQP2 p-Ser256) to total AQP2 was compared to the levels of CD81 and CD9 in both N and CKD samples. *A statistically significant difference was defined as a p-value less than 0.05, determined using a t-test.
Discussion
Exosomes can function as transporters, carrying DNA, mRNA, microRNA, and protein, and delivering these materials to targeted cells in a paracrine or endocrine fashion. As a result, they may be a promising tool for both diagnosis and therapy, and the isolation of exosomes is crucial for their application. However, two issues need to be addressed: ease of use and exosome classification [21]. To address the ease of use issue, precipitation can be employed using a high-molecular-weight polymer such as PEG. This method can be easily performed without the need for specialized equipment, and it is particularly useful for the isolation of exosomes from serum samples, which is important for diagnostic purposes. Additionally, exosome classification can be achieved using techniques such as flow cytometry, which enables the analysis of individual exosomes based on their size, surface markers, and other characteristics. Morphological analysis and biomarker blotting can also be used to confirm the identity of isolated exosomes. It is important to note that subjects' serum exosomes may belong to a population of extracellular vesicles. However, the use of a high-molecular-weight polymer in exosome isolation, as confirmed by morphological and biomarker analysis, can help address the ease of use issue. In conclusion, the potential diagnostic and therapeutic applications of exosomes can only be fully realized by addressing issues of ease of use and exosome classification. Precipitation with a high-molecular-weight polymer and techniques such as flow cytometry and biomarker analysis are effective tools that can help address these challenges and facilitate the effective use of exosomes.
Although general circulation microparticles are commonly utilized to monitor vascular dysfunction in end-stage renal failure, the presence of a definitive alteration in circulating exosomes of patients with chronic kidney disease remains uncertain. However, our study indicates that the level of serum exosomes may function as a useful parameter for the diagnosis of chronic kidney disease (as illustrated in Figure 2 and Table 2). Nonetheless, there are several other parameters that have been examined in the context of kidney-associated pathogenic conditions. For instance, research has revealed that microRNA within exosomes undergoes significant changes during diabetic nephropathy progression, and exosomal AQP2 has been identified as a biomarker for renal dysfunction. Furthermore, exosomes have been implicated in virus infections In addition to our discovery of exosome isolation, we found that the level of serum exosomes can be used as a diagnostic parameter for chronic kidney disease. We observed a significant decrease in the abundance of AQP2 on exosomes, while the phosphorylation of serine 256 on exosome-incorporated AQP2 was increased (Figure 6). These results suggest that changes in the abundance and relative phosphorylation status of AQP2 on serum exosomes are linked to the development of chronic kidney disease and could serve as a potential diagnostic marker.
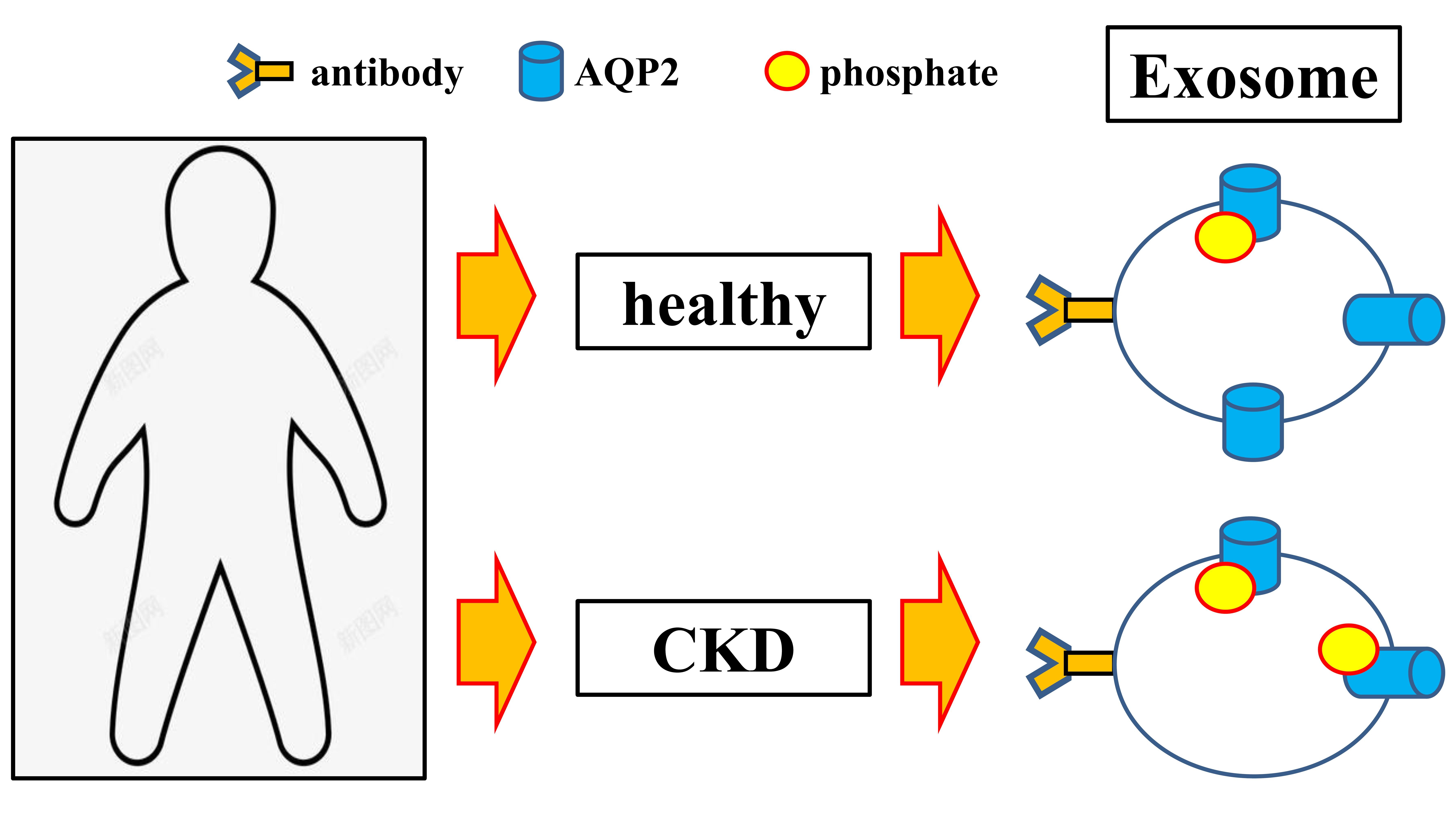
Figure 6: Exosomes, small vesicles involved in cell communication, carry molecules between cells. This study examined Aquaporin-2 (AQP2), a protein important in water balance, and its presence in exosomes, specifically in Chronic Kidney Disease (CKD). Interestingly, phosphorylation of AQP2 at a specific site was increased in CKD patients. These findings suggest that changes in AQP2 abundance and phosphorylation in exosomes may be linked to CKD development.
Although the significance of AQP2 as a biomarker for chronic kidney disease has been established, it remains unclear whether AQP2 is trafficked through exosomes. However, studies have shown that AQP2 plays a role in exosome processing through its C-terminal orientation [22]. Furthermore, the phosphorylation of serine 256 located in AQP2's C-terminal domain suggests that the phosphorylation status of AQP2 may serve as a signal involved in exosome functions. One interesting discovery is the detection of antibodies against the hepatitis virus on exosomes. This finding suggests the potential for future applications of incorporating antibodies into exosomes using biotechnological methods. However, it should be noted that this research finding is based on analysis of clinical samples, which may introduce various interfering factors. For instance, the aggregation of proteins into nanoscale-sized particles could potentially affect the accurate quantification of exosomes. Furthermore, the signaling associated with AQP2 phosphorylation needs to be further investigated using cell models to determine its role in exosomes. This is crucial for understanding the reasons behind the differential occurrence of AQP2 phosphortlation in Chronic Kidney Disease (CKD).
Conclusions
In this study, we found that the level of serum exosomes could be used as a diagnostic biomarker for chronic kidney disease. Furthermore, we observed a significant decrease in the abundance of AQP2 on the exosomes, while the phosphorylation of serine 256 on exosome-incorporated AQP2 was increased. Our findings suggest that changes in the abundance and relative phosphorylation status of AQP2 on serum exosomes are associated with the development of chronic kidney disease and could potentially be utilized as a diagnostic signal.
Author Statements
Disclosure Statement
We confirm that all authors listed on this manuscript have read and approved the final version of the manuscript, and they agree with the content and conclusions presented. Additionally, all authors have made substantial contributions to the research and the preparation of the manuscript.
Acknowledgements
We gratefully acknowledge the financial support provided by National Taiwan University Hospital Yunlin Branch (grant numbers NTUHYL107.S008 and NTUHYL109.S020) and National Taiwan University Hospital Chutung Branch (grant numbers 108001 and 109003). We also acknowledge the use of the JEM-2100F machine equipment at the Instrument Development Center of National Cheng Kung University.
Author Contributions
The research was designed by Chung Y. Te and carried out by Chung Y. Te, Kuo C. Hui, Tsai R. Yi, and Chen J. Wun. Chung Y. Te, Kuo C. Hui, and Chen J. Wun contributed to the development of new reagents and analytic tools. Data analysis was conducted by Chung Y. Te. Li W. Yi screened CKD patients, and the manuscript was written by Chung Y. Te, Li Chin, and Li W. Yi.
References
- Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015; 207: 18-30.
- Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release. 2015; 205: 35-44.
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020; 367: eaau6977.
- Gao XF, Wang ZM, Wang F, Gu Y, Zhang JJ, Chen SL. Exosomes in coronary artery disease. Int J Biol Sci. 2019; 15: 2461-70.
- Khatun M, Ray RB. Mechanisms underlying hepatitis C virus-associated hepatic fibrosis. Cells. 2019; 8: 1249.
- Nagashima S, Jirintai S, Takahashi M, Kobayashi T, Tanggis T, Nishizawa T, et al. Hepatitis E virus egress depends on the exosomal pathway, with secretory exosomes derived from multivesicular bodies. J Gen Virol. 2014; 95: 2166-75.
- Lee WC, Li LC, Ng HY, Lin PT, Chiou TT, Kuo WH, et al. Urinary exosomal microRNA signatures in nephrotic, biopsy-proven diabetic nephropathy. J Clin Med. 2020; 9: 1220.
- Oshikawa-Hori S, Yokota-Ikeda N, Sonoda H, Ikeda M. Urinary extracellular vesicular release of aquaporins in patients with renal transplantation. BMC Nephrol. 2019; 20: 216.
- Amabile N, Guérin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, et al. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005; 16: 3381-8.
- García-Romero N, Madurga R, Rackov G, Palacín-Aliana I, Núñez-Torres R, Asensi-Puig A, et al. Polyethylene glycol improves current methods for circulating extracellular vesicle-derived DNA isolation. J Transl Med. 2019; 17: 75.
- Brittain GC, Chen YQ, Martinez E, Tang VA, Renner TM, Langlois MA, et al. A novel semiconductor-based flow cytometer with enhanced light-scatter sensitivity for the analysis of biological nanoparticles. Sci Rep. 2019; 9: 16039.
- Patel GK, Khan MA, Zubair H, Srivastava SK, Khushman M, Singh S, et al. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci Rep. 2019; 9: 5335.
- Lee SS, Won JH, Lim GJ, Han J, Lee JY, Cho KO, et al. A novel population of extracellular vesicles smaller than exosomes promotes cell proliferation. Cell Commun Signal. 2019; 17: 95.
- Roche JV, Survery S, Kreida S, Nesverova V, Ampah-Korsah H, Gourdon M, et al. Phosphorylation of human aquaporin 2 (AQP2) allosterically controls its interaction with the lysosomal trafficking protein LIP5. J Biol Chem. 2017; 292: 14636-48.
- Sakai M, Yamamoto K, Mizumura H, Matsumoto T, Tanaka Y, Noda Y, et al. Phosphorylation profile of human AQP2 in urinary exosomes by LC-MS/MS phosphoproteomic analysis. Clin Exp Nephrol. 2020; 24: 762-9.
- Adams A. Concentration of Epstein-Barr virus from cell culture fluids with polyethylene glycol. J Gen Virol. 1973; 20: 391-4.
- Jung MK, Mun JY. Sample preparation and imaging of exosomes by transmission electron microscopy. J Vis Exp. 2018; 56482.
- Sosa-Jurado F, Hilda Rosas-Murrieta N, Guzman-Flores B, Perez Zempoaltecalt C, Patricia Sanchez Torres A, Ramirez Rosete L, et al. Prevalence of serologic hepatitis B markers in blood donors from Puebla, Mexico: the association of relatively high levels of anti-core antibodies with the detection of surface antigen and genomic DNA. Hepat Mon. 2016; 16: e36942.
- Wang JH, Chen CH, Chang CM, Feng WC, Lee CY, Lu SN. Hepatitis C virus core antigen is cost-effective in community-based screening of active hepatitis C infection in Taiwan. J Formos Med Assoc. 2020; 119: 504-8.
- Dad HA, Gu TW, Zhu AQ, Huang LQ, Peng LH. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol Ther. 2021; 29: 13-31.
- Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020; 10: 3684-707.
- Clarke-Bland CE, Bill RM, Devitt A. Emerging roles for AQP in mammalian extracellular vesicles. Biochim Biophys Acta Biomembr. 2022; 1864: 183826.