
Review Article
Ann Depress Anxiety. 2023; 10(1): 1117.
Noninvasive Vagal Nerve Stimulation for Opioid Use Disorder
J Douglas Bremner, MD1,2,3*; Asim H Gazi, BS4; Tamara P Lambert, MPH, MEng5; Afra Nawar, BS4; Anna B Harrison, MS4; Justine W Welsh, MD1; Viola Vaccarino, MD, PhD6,7; Kevin M Walton, PhD8; Nora Jaquemet, BS1; Kellen Mermin-Bunnell, BS1; Hewitt Mesfin, BS1; Trinity A Gray, BS1; Keyatta Ross, BS1; Georgia Saks BS5; Nikolina Tomic, MS4; Danner Affadzi, BS1; Marom Bikson, PhD9; Amit J Shah, MD, MSCR3,6,7; Kelly E Dunn, PhD10; Nicholas A Giordano, PhD, RN11; Omer T Inan, PhD4,5
1Department of Psychiatry & Behavioral Sciences, Emory University School of Medicine, Atlanta GA
2Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta GA
3Atlanta Veterans Affairs Healthcare System, Decatur GA
4School of Electrical and Computer Engineering, Georgia Institute of Technology, Atlanta, GA
5Coulter Department of Biomedical Engineering, Georgia Institute of Technology, Atlanta, GA
6Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, GA
7Department of Medicine, Division of Cardiology, Emory University School of Medicine, Atlanta GA
8Clinical Research Grants Branch, Division of Therapeutics and Medical Consequences, National Institute on Drug Abuse, Bethesda, MD
9Department of Biomedical Engineering, The City College of New York, New York, NY
10Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore MD
11Nell Hodgson Woodruff School of Nursing, Emory University, Atlanta, GA
Corresponding author: J Douglas Bremner, MD Department of Psychiatry, 12 Executive Park DR NE, Rm 333, Atlanta, GA Tel: (404) 712-9569-8083 Email: jdbremn@emory.edu
Received: June 29, 2023 Accepted: July 26, 2023 Published: August 02, 2023
Abstract
Background: Opioid Use Disorder (OUD) is an escalating public health problem with over 100,000 drug overdose-related deaths last year most of them related to opioid overdose, yet treatment options remain limited. Non-invasive Vagal Nerve Stimulation (nVNS) can be delivered via the ear or the neck and is a non-medication alternative to treatment of opioid withdrawal and OUD with potentially widespread applications.
Methods: This paper reviews the neurobiology of opioid withdrawal and OUD and the emerging literature of nVNS for the application of OUD. Literature databases for Pubmed, Psychinfo, and Medline were queried for these topics for 1982-present.
Results: Opioid withdrawal in the context of OUD is associated with activation of peripheral sympathetic and inflammatory systems as well as alterations in central brain regions including anterior cingulate, basal ganglia, and amygdala. NVNS has the potential to reduce sympathetic and inflammatory activation and counter the effects of opioid withdrawal in initial pilot studies. Preliminary studies show that it is potentially effective at acting through sympathetic pathways to reduce the effects of opioid withdrawal, in addition to reducing pain and distress.
Conclusions: NVNS shows promise as a non-medication approach to OUD, both in terms of its known effect on neurobiology as well as pilot data showing a reduction in withdrawal symptoms as well as physiological manifestations of opioid withdrawal.
Introduction
Opioid Use Disorder (OUD) is a national epidemic with devastating consequences. Deaths from opioid overdose, including prescription opiates like OxyContin and Percocet, as well as heroin and other illegal opiates, increased five-fold from 1999 to 2016 [1]. Opioid overdose is now the leading cause of accidental death in the United States [2]. In 2021, there were over 100,000 drug overdose-related deaths, most of which were due to opioids [3]. The standard of care for treating Opioid Use Disorder (OUD) are Medications for Opioid Use Disorder (MOUD), which includes opioid receptor agonists and antagonists; however, the barriers to MOUD care are high, including lack of access to MOUDs with proven benefit, like opioid agonists, methadone and buprenorphine [4-9]. Most OUD patients will relapse without medication, and about half relapse even after treatment with the gold standard opioid-agonist, methadone [10]. Many patients don’t want to take opioid agonists, but treatment with naltrexone, an opioid receptor antagonist, requires an extended period of detoxification during which the risk of relapse and overdose-related death is high [11-13]. During this period of detoxification there is an increase in both the probability of relapse (due to withdrawal symptoms) and the risk of overdose-related death due to a loss of tolerance [4,14,15]. Management of withdrawal during the treatment of OUD is crucial when initiating OUD treatment protocols that will maintain long-term efficacy in prevention of relapse. Non-pharmacological methods that treat withdrawal could provide a bridge to facilitate effective implementation and continuation of opioid antagonist pharmacological therapies. Additionally, the initial induction period when opioid agonist dose adjustment occurs is often associated with symptoms of opioid withdrawal that can increase the risk of relapse and/overdose. Additional interventions to use as adjuncts to FDA-approved treatments of opioid withdrawal, such as lofexidine [16-18], will also be useful. This paper describes the use of a form of neuromodulation called Non-invasive Vagal Nerve Stimulation (nVNS), and outlines its potential usefulness for the treatment of OUDs in the context of its effects on neurobiology and how this may intersect with the neurobiology of OUD and opioid withdrawal.
Changes in Neurobiological Systems in OUD
OUD is characterized by alterations in multiple neurobiological systems [19]. Opioid withdrawal is associated with uncomfortable symptoms including headache, nausea and vomiting, diarrhea, sweating, fatigue, anxiety, and sleep disturbance. These symptoms are driven by activation of the sympathetic nervous system with release of inflammatory biomarkers and alterations in dopaminergic reward systems that play a critical role in both addiction and relapse risk [20]. Mesocortical and mesolimbic dopaminergic pathways from the ventral tegmentum area to the medial prefrontal cortex and ventral striatum (nucleus accumbens) play an important role in OUD, and stress and substance use have similar effects on these systems [21-30]. Repeated exposure to opioids, both prescription and illicit, among individuals living with OUD are linked to increased risks for developing pain sensitivity and centralization due, in part to, increased activation of NMDA receptors leading to sensitization of spinal neurons [31]. Compared to controls, persons with OUD show increased cortisol, heart rate and blood pressure response to both drug cues [32] and to a laboratory-based stress task involving public speaking [33]. Persons with OUD have also shown increased drug craving after exposure to drug cues compared to controls [32] although laboratory-based mental stress did not potentiate drug craving over exposure to drug cues alone [33]. Women with OUD showed a pattern of lower cortisol response and experienced more subjective stress during public speaking than men with OUD [34]. Patients with PTSD and either cocaine or alcohol substance use disorders showed increased drug craving following both trauma and drug-related cues [35]. Patients with PTSD experience higher pain levels and have increased instance of substance use disorders than non-PTSD patients, although pain is not a mediator of the increased substance use rates in these patients [36]. These studies show that drug cues activate neurobiological systems that underlie craving, and that responses to this activation differ by gender.
Treatment of OUD with MOUDs
Standard treatments for OUD are medications that act on opioid receptor, including buprenorphine, methadone, and naltrexone. Methadone is a long-acting full opioid agonist of the mu opioid receptor. Buprenorphine is a partial agonist of the mu opioid receptor and an antagonist of the kappa opioid receptor. Methadone must be administered in a specialized clinic, and access to buprenorphine, although it is effective in reducing the risk of relapse and overdose and can be taken at home, is limited. A recent “secret shoppers” study in which volunteers called medical clinics seeking treatment for an OUD showed that only 27% of individuals were able to be seen in a clinic and obtain a buprenorphine prescription if they were not willing to pay cash for treatment [7]. Fifty percent of clinics listed as available for buprenorphine treatment either did not have a working phone number or a physician or practitioner with the necessary specialized certifications to prescribe, and of those, 30% outright refused to treat non-cash paying patients [7]. Thus, buprenorphine treatment, with costs upwards of $350, is cost prohibitive for most people, further precluding access for OUD patients in need of care [9].
Naltrexone is an antagonist of the opioid receptor that is an alternative to opioid agonist therapy that is also effective for OUD [5,11,15,37,38]. Long--acting naltrexone is superior to treatment as usual including non-pharmacological counseling for relapse prevention [12]. Once safely initiated, long-acting naltrexone has equivalent or increased efficacy in relapse prevention as compared to buprenorphine [13]. Naltrexone is an effective long-term treatment for OUD, but the required opioid abstinence and subsequent withdrawal period prior to initiation represents a key barrier to treatment [39]. Interventions during the period of opioid withdrawal to reduce symptoms and prevent relapse, allowing patients to complete the period of abstinence required before the initiation of long-acting naltrexone treatment may both benefit patients with OUD in recovery maintenance and reduce overdose-related deaths during this critical period [39].
Neuroimaging and Neurobiology of OUD
Advancements in neuroimaging have allowed for identification of brain regions and specific neural circuits that are implicated in OUD [40]. The major pathways that play an important role in addiction and relapse are dopaminergic mesolimbic and mesocortical pathways from the ventral tegmentum to the ventral striatum (nucleus accumbens) and medial prefrontal cortex (anterior cingulate) [20,41] and the amygdala [40,42,43]. Release of dopamine in the nucleus accumbens (ventral striatum) is associated with pleasure and reward and is accepted as playing a role in addiction [19,20,44-47]. The medial prefrontal cortex plays an important role in modulation of emotion that is relevant to relapse and addiction [40,42,43] as well as drug craving. The amygdala mediates anxiety and fear reactions and plays a key role in OUD [40,42,43].
The maintenance of addiction and relapse is often related to craving precipitated by conditioned responses to exposure to drug cues in patients with OUD [48]. These neural circuits allow for the development and maintenance of such conditioned responses in patients with OUD [40,42,43]. The amygdala likely plays a role in symptoms of anxiety associated with opioid addiction and withdrawal [49]. The medial prefrontal cortex is implicated in the appraisal and regulation of emotions. By inhibiting amygdala activity through extinction mechanisms [50] the medial prefrontal cortex plays a critical role in the acquisition of fear memories as shown in both animal studies [51,52] and brain imaging studies in humans [53-63], highlighting its role in anxiety which is a part of the opioid withdrawal response [19]. Changes in these brain areas are reversible with treatment in patients with OUD [46].
The Locus Coeruleus (LC), located in the pons, is the major source of noradrenergic cell bodies in the brain [64]. Noradrenergic neurons project to multiple areas in the brain, including amygdala, medial prefrontal cortex, hippocampus, ventral striatum, and other areas of the cerebral cortex [64]. Release of norepinephrine, which occurs under both stress and opioid withdrawal, causes an increase in attention and vigilance behaviors, and activates the cardiovascular system [23,24,64]. Inputs to the LC come from the nucleus paragigantocellularis and nucleus prepositus hypoglossi, and endogenous opiates in this brain area are inhibitory to LC neurons [65,66]. Withdrawal of opioids in animals addicted to methadone causes a rebound increase in LC activity, resulting in increased norepinephrine release in the amygdala that subsequently activates the cardiovascular system and drives symptoms of withdrawal [65]. Opioids in the LC initially inhibit of production of adenylyl cyclase and Cyclic Adenosine Monophosphate (cAMP) [67,68]. With prolonged dependence there is eventually an increase in adenylyl cyclase and cAMP resulting in increased cAMP dependent response element binding protein (CREB). This upregulation likely represents a compensatory mechanism for the initial inhibitory effects of opioids [67,68]. Upregulation in the cAMP cascade in the LC is hypothesized to represent a component of tolerance, as well as the mechanism of opioid dependence and withdrawal [67,68]. Norepinephrine and the LC therefore play a central role in opioid addiction and symptoms of withdrawal due to rebound sympathetic activation.
Positron Emission Tomography (PET) studies in patients with OUD implicate similar neurobiological systems and circuits as seen in animal models [46,69]. Morphine acutely reduces brain metabolic activity in the superior frontal gyrus, paracentral lobule, supramarginal gyrus, anterior cingulate, caudate (striatum), gyrus rectus, and middle temporal gyrus [70]. Functional Magnetic Resonance Imaging (fMRI) studies showed abnormal connectivity in OUD in ventral striatum, medial prefrontal cortex (anterior cingulate), amygdala and insula [71]. Functional imaging studies of brain blood flow and metabolism using PET showed increased activation of ventral striatum and medial prefrontal cortex (anterior cingulate) during opioid [46] and nicotine [72,73] craving. Patients with OUD who are exposed to emotional pictures during heroin abstinence had increased connectivity as measured with resting state fMRI between left amygdala and left fusiform gyrus and right amygdala and right orbitofrontal cortex, this normalized after administration of heroin [74].
Chronic opioid use is associated with a decrease in binding of the Dopamine Transporter (DAT) [75] and D-2 receptor binding measured with PET and the radiolig and [C-11] raclopride [40,69,76]. This may reflect a decrease in endogenous dopamine release following chronic overstimulation with opioids and/or decreased transporter and/or receptor binding [40]. DAT uptake was not associated with heroin craving in abstinent OUD patients [75]. These findings implicate dopaminergic pathways in the ventral striatum and medial prefrontal cortex in OUD.
Effects of Early Trauma on Vulnerability to OUD
Early life trauma plays a critical role in the development and maintenance of OUD in many patients [77-83]. Individuals with a high number of Adverse Childhood Experiences (ACEs) have a 10-fold increase in risk for the use of injected drugs, mostly opioids [77,84,85], and there are important links between OUD and Posttraumatic Stress Disorder (PTSD) [36,79,80,86-93]. Recurrent stressors and exposure to triggers of traumatic memories in everyday life is a common precipitator of relapse in patients with OUD [22,89,94], especially for women using prescription opioids [95]. There is a great deal of overlap in brain circuits mediating OUD and PTSD (Figure 1). The fact that nVNS modulates many of these brain regions suggests that it could be useful for both disorders as well as the complex co-morbid conditions that are often seen in patients with OUD.
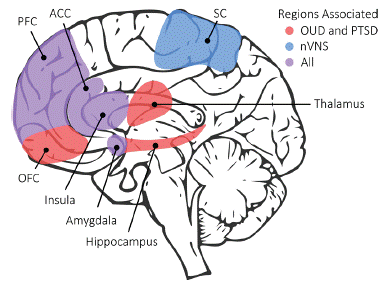
Figure 1: Neural circuits in Opioid Use Disorder (OUD) and Posttraumatic Stress Disorder (PTSD) and effects of Non-invasive Vagal Nerve Stimulation (nVNS). OUD and PTSD share overlapping brain circuits (hence the association of trauma with OUD) including Orbitofrontal Cortex (OFC), thalamus and hippocampus (red colored regions in diagram) as well as areas also impacted by nVNS like Prefrontal Cortex (PFC), Anterior Cingulate Cortex (ACC), insula and amygdala. nVNS additionally has effects on Somatosensory Cortex (SC).
Vagal Nerve Stimulation for OUD
Neuromodulation represents a new paradigm in the field of mental and substance use disorders [96,97]. Vagal Nerve Stimulation (VNS), is an electrical stimulation treatment that may be useful in the treatment of OUD given its demonstrated effects on brain circuits and systems implicated in OUD and opioid withdrawal and its demonstrated positive effects on related symptom areas like pain and anxiety [96-103]. Currently, implantable VNS devices are FDA-approved to treat medically refractory epilepsy and treatment-resistant depression [104,105]. Neuromodulation using VNS can reduce symptoms of pain [106,107] and anxiety [108,109] both of which contribute to the development of OUD and/or precipitate relapse. VNS is also beneficial in the treatment of headaches [110-113], epilepsy [114-119] and major depression [104,105,120-131]. The effects of VNS on neuroplasticity and learning suggest it may be useful in extinction of the conditioned stimulus and reinforcement processes that underlie addictions [132-138] possibly through enhancement of Long Term Potentiation (LTP) in the hippocampus [139]. VNS reduces autonomic tone and sympathetic function [140-145] immune function [146-153] and fear circuits that likely play a role in symptoms of anxiety associated with OUD [108,154-159], all of which indicate its potential to play a beneficial role in OUD and withdrawal. Animal studies show that VNS enhances neuroplasticity in the frontal cortex and reduces cocaine seeking behaviors [160]. In animal models of schizophrenia, VNS enhances mesofrontal dopamine transmission [161]. Recent studies have also applied VNS to the treatment of schizophrenia [162,163], obsessive-compulsive disorder [164], PTSD [165-167] and Mild Traumatic Brain Injury (mTBI) [165], all with promising results. The first generation of VNS devices had to be surgically implanted, reducing their feasibility in treatment of mental disorders [102]. Clinical trials testing efficacy of these devices were limited by cost, inconvenience, potential complications, the inability to do placebo comparisons related to ethical considerations [102], and the lack of insurance reimbursement [168,169]. The failure of Medicare and therefore other insurance agencies to reimburse for either VNS implantation or necessary followup care has been a major limitation to its wide-spread use [169].
However, a new generation of non-invasive devices holds potential for wide-spread implementation and is more amenable to rigorous placebo-controlled research studies [97,102]. GammaCore, a Non-invasive VNS (nVNS) that delivers electrical stimulation to the vagus nerve via the neck, was recently approved by the Food and Drug Administration (FDA) for the treatment of intermittent cluster headache [113] and shows promise for wide-spread implementation in psychiatry due to low cost and convenience [102]. We have shown that Transcutaneous Cervical VNS (tcVNS) using the gammaCore blocks sympathetic responses to stress in traumatized persons with and without PTSD [170-177], and blocks inflammatory (IL-6, IFN-γ) [(178] and neuropeptide (Pituitary Adenylate Cyclase Activating Peptide, PACAP) [179] responses to stress in PTSD. It also modulates brain areas involved in emotion in traumatized persons with [180] and without [181] PTSD and reduces PTSD symptoms [182]. Based on our findings of reduction in PTSD symptoms with tcVNS versus sham stimulation after three months of twice daily treatment [182] the FDA granted Breakthrough Device Designation for the gammaCore for PTSD on January 11, 2022. VNS blocks the sympathetic, cardiovascular, and inflammatory activation associated with opioid withdrawal and modulates central brain regions involved in anxiety and reward systems, has the potential to facilitate conversion to long-term therapies such as opioid receptor antagonists and prevent relapse during the critical, potentially lethal, withdrawal period [47,183].
Physiological Correlates of Vagal Nerve Stimulation
The vagus nerve originates in the brain stem and has fibers that travel to both central brain areas as well as peripheral organs [184,185]. Mostly afferent projections of myelinated A fibers of the vagus, relay sensory activity of the visceral organs to the brain through the Nucleus Tractus Solitarius (NTS) in the medulla oblongata. Efferent branches of mostly unmyelinated C fibers of the vagus nerve modulate autonomic and inflammatory function [152] through peripheral anti-sympathetic, pro-parasympathetic, and anti-inflammatory effects [185-187]. These effects may benefit patients with OUD [147,188].
The effects of nVNS, however, are likely mediated through the brain [189]. Projections of the vagus to the brain through the Nucleus Tractus Solitarius (NTS) extend to the locus ceoruleus and hypothalamus [187], key areas involved in sympathetic hyperarousal during opioid withdrawal, as well as to brain areas such as the amygdala and anterior cingulate that mediate emotion and anxiety [187]. Afferent vagal nerve fibers travel through the carotid sheath in the neck (cervical) just medial to the sternocleidomastoid muscle [190] as well as in the ear (auricular), and devices have been developed to non-invasively stimulate the vagus nerve at either branch.
VNS has effects on a number of neurotransmitters that mediate symptoms of mental disorders including OUD [191]. The NTS has direct projections to the Locus Coeruleus (LC), which is located in the pons and is the major site of Norepinephrine (NE) cell bodies in the brain [187]. One of the primary effects of VNS on the brain is activation of norepinephrine in the LC. VNS acts through the LC to increase norepinephrine release in the medial prefrontal cortex, amygdala and hippocampus [192-195], an effect mediated by vagal afferent fibers [193]. Increased NE has a secondary effect on the serotonin (5HT) system through excitatory alpha-1 adrenoreceptors that increase 5HT in the dorsal raphe [192,196-198]. Chronic VNS treatment increases the firing rates of norepinephrine neurons in the LC, hippocampus, and prefrontal cortex and of serotonergic neurons in the dorsal raphe but not in the hippocampus or prefrontal cortex [197,199]. VNS increases both the firing rate and burst pattern of NE neurons [192], an effect which can be blocked by the muscarinic acetylcholine antagonist scopolamine [200]. Chronic VNS decreases dopamine neuronal firing in the ventral tegmental area but leads to increases in extracellular dopamine in the nucleus accumbens and prefrontal cortex [161,197]. Two months of VNS treatment raises levels of dopamine and serotonin metabolites in the Cerebrospinal Fluid (CSF) of patients with epilepsy [142]. Thus, VNS has similar effects to antidepressants [192] without the autoreceptor desensitization seen with chronic use of these medications [192,197].
VNS also has effects on learning and memory systems that may be relevant to stimulus reinforcement and stress diathesis theories of addiction [19,154,201]. Hormones and neurotransmitters released during stress, including cortisol and epinephrine, have effects on learning and memory [198,202-207] even though they don’t cross the blood-brain barrier [208]. One possible explanation for this phenomenon is that the vagus nerve transmits this information to the brain [209]; in fact, lesions of the vagus nerve block the effects of peripheral hormones and neurotransmitters on memory [208,209]. VNS enhances memory retention in a U-shaped curve, with maximum effect at 0.4 mA in rat models [210], an effect mediated through afferent vagus pathways and not efferent pathways [211]. VNS acts through the LC to increase NE in the basolateral nucleus of the amygdala (BLA) to concentrations capable of modulating emotional memory [193]. Its enhancement of synaptic transmission [212,213], Long-Term Potentiation (LTP) [139], and neurogenesis [214] in the hippocampus likely accounts for its effects on learning and memory as well as its anti-depressant effects (215-217). When paired with conditioned cues, VNS also facilitates extinction in animal models of classic fear conditioning [156-158,218] and reduces fear-like behaviors [156,218]. This effect is mediated through enhancement of the infralimbic (medial prefrontal) amygdala (basal nucleus of the amygdala) pathway [158].
VNS plays a role in neural plasticity and autonomic nervous system function that are relevant to production and maintenance of addictions as well as reinforcement and reinstatement of drug use [159,191,219,220]. Evidence for the role of VNS in neural plasticity comes from animal models showing beneficial effects in treatment of tinnitus when paired with an auditory tone [132-135], recovery of cognitive function after stroke [136], and motor movement when paired with training [221-224]. Animal studies show that VNS reduces ventricular arrhythmia in the setting of myocardial ischemia [225] and promotes recovery from cerebral hemorrhage [226] congestive heart failure [227] and other cardiovascular events [219,228]. VNS promotes learning and memory in patients with Alzheimer’s Disease [229,230] and acts through the LC in animal models of TBI to enhance new learning and memory, synaptic plasticity and motor recovery [231,232]. In summary, VNS has a broad range of effects on neural circuits and symptoms that likely are beneficial for patients with OUD, including opioid withdrawal.
The anti-inflammatory and anti-stress hormone effects of VNS may be beneficial for alleviating symptoms of opioid withdrawal. Opioids activate the Hypothalamic-Pituitary-Adrenal (HPA) axis (which through cortisol plays a critical role in stress) and inflammatory systems, and dysregulation of these systems during opioid withdrawal likely plays a key role in the symptoms of withdrawal in OUD patients [233]. VNS has modulatory effects on the HPA axis which may benefit symptoms of withdrawal [47,234-236]. Repeated administration of opioids, including morphine, in animal models activates inflammasomes [237] including p38 Mitogen-Activated Protein Kinase (MAPK), nuclear factor-κB (NF-κB) p65 and Nod-like Receptor Protein (NLRP3) [237]. Opioids also lead to increases in the inflammatory factors interleukin-6 (IL-6) [183,233,238] and Tissue Necrosis Factor- α (TNF- α) [239], as well as corticosterone (cortisol in humans) [233]. Opioid dependence is associated with increases in IL-1β, IL-2, and IL-8 [183]. Stress, which is often linked to OUD, is associated with increases in IL-1B, IL-6, TNF- α, Interferon Gamma (IFNγ) and C Reactive Protein (CRP) in animal models [240,241], and we have shown an increase in mental stress-induced IL-6 and IFNγ in PTSD patients [178,182,242]. Opioid-addicted animals show greater increases in IFNγ [243] and TNF-α when under stress than non-addicted animals [239], and post-mortem studies show increased TNF-α in the LC of patients with OUD [244,245]. These inflammatory markers interacting with the HPA axis may mediate, in part, symptoms of withdrawal in OUD and contribute to the development and maintenance of addictions [240,241,246]. VNS counters these inflammatory responses to opioids, through a reduction of high mobility group protein B 1 (HMGB1) [237,247,248], TNF-α production [249], RANTES (CCL5) (250), Macrophage Inhibition Factor (MIF) [214,251,252], and kynurenines [153,245,249,253,254]. Emerging evidence indicates the anti-inflammatory effects of VNS seen in response to opioids may also benefit individuals living with co-occurring chronic pain [255]. These studies demonstrate the potential for VNS to have utility in opioid withdrawal symptom reduction through its modification of immune, neuropeptidal and neurohormonal systems. Some key questions remain to be addressed by future research, including why VNS acts through the locus coeruleus to stimulate release of norepinephrine in the hippocampus while simultaneously reducing sympathetic function in the periphery.
Effects of Vagal Nerve Stimulation on Brain Function: Relevance to Mental Disorders and Addictions
Studies have begun to map neural correlates of VNS, improving understanding of potential mechanisms of action in OUD [256-258]. Functional imaging studies in human subjects show that VNS, as predicted from animal models, results in effects on brain regions connected to the Nucleus Tractus Solitarius (NTS), including the medial prefrontal cortex (anterior cingulate) and amygdala [259-261]. Successful treatment of depression with VNS also results in changes to brain regions implicated in that disorder [262-265] as well as beneficial effects on cardiovascular function [266] and cognition [131]. These findings from studies of implanted VNS devices have been replicated in functional imaging studies of healthy human subjects with non-invasive vagal nerve stimulation applied both through the ear [267] and the neck [268,269]. We have shown that tcVNS blocks insula response to stress and enhances anterior cingulate function in PTSD [180,181].
In 2017, the FDA approved a percutaneous form of VNS for the treatment of opioid withdrawal [270]. This was based on the results of an open label trial of 73 patients with OUD, where significant reductions in opioid withdrawal symptoms were observed within one hour of VNS treatment initiation [271]. This study did not use a sham control or obtain objective physiological measurements of sympathetic nervous system activity. Other studies have shown that transcutaneous auricular VNS (taVNS, applied to the ear) is beneficial in promoting oral feeding in infants with brain damage [272] and in withdrawal from opioids in infants born addicted to opioids [273].
Transcutaneous Cervical Vagal Nerve Stimulation for Acute Opioid Withdrawal
To further evaluate the clinical uses of VNS, we assessed the effects of tcVNS on opioid withdrawal in 21 patients with OUD. Patients were randomized to a double-blind administration of active tcVNS (N=10), or sham stimulation (N=11) paired with viewing of neutral and opioid use videos designed to elicit opioid craving (Figure 2). Participants stopped using opioids the night before experimentation and were studied prior to initiation of medication for their OUD. Each administration of vagal nerve stimulation occurred two minutes. There were a total of six stimulations per subject. Subjective opioid withdrawal and craving as well as pain and anxiety were measured using numerical rating scales with a range of 0 to 10 with 10 being most severe. Perceived distress was measured due its role in craving and relapse [88,274-276] using the Subjective Units of Distress Scale (SUDS) which rates subjective distress on a scale of 0 to 100 [277]. Peripheral autonomic activity was measured using wearable sensing devices [278]. Active tcVNS compared to sham stimulation resulted in significant reductions in withdrawal (-1.9±3.7 versus 0.4±1.0, p=.047) [279] and pain [280] (-0.8±2.4 versus 0.9±1.0, p=.045) as well lower opioid craving which reached trend significance (-2.2±3.6 versus 0.1±2.7, p=0.1) [279] (Table 1). tcVNS also resulted in significant decreases in subjective distress measured with the SUDS compared to sham stimulation (-17.5±26.5 versus 2.2±5.9, p=.004). Active tcVNS also had an effect on peripheral autonomic function measured with a significant reduction in heart rate measured with wearable sensing devices (-5.5±3.5 bpm versus -1.4±4.6 bpm, p=.035) [279] as well as a reduction in breathing irregularity (i.e., respiratory variability) (-368.5±514.1 ms versus 74.2±253.1 ms, p=.02) [281]. Another finding of this study was that opioid withdrawal was associated with an increase in sinusoidal movements in the peripheral extremities that were associated with increased severity of subjective opioid withdrawal [282]. These findings support adjunctive use of tcVNS in mitigating acute opioid withdrawal symptoms, especially during detoxification and transitional periods (e.g., prior to MOUD) – periods of increased vulnerability [4] when opioid withdrawal symptoms can be highly uncomfortable and precipitate relapse in patients with OUD [283-287].
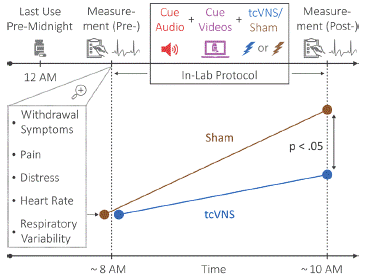
Figure 2: Study protocol for Transcutaneous Cervical Vagus Nerve Stimulation (tcVNS) in Opioid Use Disorder (OUD). Patients with OUD discontinued use of opioids by midnight of the night before the protocol. They presented early in the morning before initiation of Medication for Opioid Use Disorder (MOUD) initiation and underwent measurement of physiological variables with wearable sending devices (heart rate, respiratory variability) and behavioral variables with rating scales (withdrawal, pain, distress). tcVNS or sham was paired with opioid use cues (videos) in a double blind randomized fashion. There were significant increases in behavioral and physiological measures of withdrawal in the sham versus tcVNS group (i.e. tcVNS blocked measures of opioid withdrawal both subjective and objective related to sympathetic nervous system function).
Outcome
Timepoint
Active (n=10)
Sham (n=11)
VAS-Withdrawal
Pre
5.8±3.1
5.3±2.5
Post
3.9±3.1*
6.3±2.5
VAS-Craving
Pre
7.4±2.7
6.8±2.5
Post
5.2±3.4
6.9±3.5
VAS-Anxiety
Pre
5.6±3.6
6.4±2.3
Post
3.7±3.0*
6.3±2.6
SUDS
Pre
43.1±32.3
45.1±24.5
Post
25.6±30.4*
47.6±28.5
NRS-Pain
Pre
3.5±2.6
4.4±1.7
Post1
2.4±2.5*
5.3±2.3
COWS Total
Pre
7.6±3.4
8.5±3.1
Post
8.2±4.6
10.3±4.2
Abbreviations: VAS, Visual Analog Scale; SUDS, Subjective Units of Distress Scale; NRS, Numerical Rating Scale; COWS, Clinical Opiate Withdrawal Scale. *p<0.05 for reduction post treatment in the active but not the sham stimulation group. 1Missing data for one participant in the active group. Adapted from Gazi et al 2022 [279,280].
Table 1: Active tcVNS and Sham Group Behavioral Measures Before and After Stimulation in Patients with OUD in active Opioid Withdrawal.
Avoidance of withdrawal symptoms, with associated in increases in pain and anxiety, is often cited as a primary factor in continuing opioid use [283]. Pain intensification during opioid withdrawal has been independently associated with relapse risk [288,289]. For many patients, opioid treatment of a primary pain disorder represents an entry phase into opioid addiction. The return of pain either from the primary pain disorder that was previously masked by opioid use or a new injury in a patient recovering from OUD with an increased pain sensitivity due to the withdrawal state can motivate opioid use, thus perpetuating the addictive cycle [290,291]. Even mild pain can catalyze the learned associations between pain and drug relief among individuals with chronic pain and OUD [292]. Further, increased pain sensitivity and undermanaged pain are associated with worst treatment outcomes, including relapse, for patients with co-occurring chronic pain and OUD [293]. Anxiety and distress can also trigger relapse [88,274,275,287]. Patients with OUD will often continue to use opioids to avoid negative affective states [283,287], which as outlined above are often linked to a history of trauma [79,80]. Affective distress can trigger relapse, even after lengthy periods of abstinence [276,294]. Continued use of VNS into the period of recovery when withdrawal symptoms are reduced but still present in diminished form may reduce the risk of relapse in OUD patients.
Opioid craving and withdrawal are mediated by complex interaction of brain and peripheral autonomic and inflammatory processes in response to the withdrawal of opioids [19,285,294]. The extended amygdala, prefrontal, hypothalamic, and brainstem regions are postulated to mediate dysphoria and autonomic imbalance associated with opioid withdrawal [19,285,294], brain regions which are also implicated in PTSD and other stress-related disorders [295,296].
The effects of tcVNS on signs of opioid withdrawal were greatest for heart rate. Heart rate is mediated by both the parasympathetic and sympathetic branches of the autonomic nervous system, where decreases in sympathetic arousal and/or increases in parasympathetic activity cause heart rate to decrease [65,297]. Subjective withdrawal is also driven by factors including overdrive of the locus coeruleus/noradrenergic/sympathetic nervous system [65]. Symptoms of withdrawal including increased heart rate, sweating, and respiration, and pupil constriction, are driven by increased sympathetic arousal and/or decreased parasympathetic activity [65,68], a similar physiology and presentation to that of patients with stress-related psychiatric disorders [23,24,298,299]. Successful interventions with tcVNS would be expected to reduce both autonomic signs as well as behavioral symptoms in both disorders by intervening at the level of the autonomic nervous system.
Conclusions
Acute opioid withdrawal in the context of opioid addiction in patients with OUD is related to a complex interaction of central brain regions involved in emotion and peripheral autonomic and inflammatory processes. Non-invasive Vagal Nerve Stimulation (nVNS) non-pharmacological intervention that offers a promising new tool in the treatment of opioid withdrawal. Safety is demonstrated by successful administration in thousands of patients with a range of disorders from mTBI to depression, PTSD and OUD without adverse effects. Adjunctive use of nVNS and medication management of opioid withdrawal may be useful when establishing long-term treatment for OUD with opioid agonists including methadone and buprenorphine, or during the abstinence period before the initiation of treatment with opioid antagonists like naltrexone treatment, reducing symptoms and enhancing the potential for a successful recovery [284]. The non-invasive, non-pharmacological nature of nVNS as well as its ease of use in the home and ability to self-administer during periods of vulnerability make it a useful tool to reduce the risk of opioid relapse by decreasing withdrawal symptoms, pain, and distress. NVNS may be in particular be a useful tool in the window of heightened vulnerability to relapse during the initial period of abstinence [38]. NVNS poses minimal risk, requires minimal training for self-administration and has the potential to be a widely-applied, effective treatment for patients with OUD due to lower cost and enhanced convenience [300].
Author Statements
Disclosures
The active and sham vagus nerve stimulation devices used in this research were provided in-kind by electroCore, Inc. JDB has received grant funding support in the past from electroCore and currently serves on the Scientific Advisory Board for Evren Technologies, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Acknowledgements
A.H.G. was supported by a National Science Foundation Graduate Research Fellowship (DGE-2039655). T.P.L. was supported by NIDA Diversity Supplement UG3 DA048502-S2 and R.A.O. by NIDA Summer Internship Supplement UG3 DA048502-S1. The research reviewed here was supported by the National Institutes of Health (UG3 DA048502). N.A.G. was supported by NIDA K23DA057415. JDB was supported by R01 MH120262 and VA Merit I01 RX003418 and I01 CX002331. Additional support came from R01s HL155711, HL136205, HL109413 and T32 HL130025. The authors gratefully acknowledge the Alliance Recovery Center in Decatur, GA for their invaluable assistance with recruitment efforts.
References
- Buchanich JM, Balmert LC, Williams KE, Burke DS. The effect of incomplete death certificates on estimates of unintentional opioid-related overdose deaths in the United States, 1999-2015. Public Health Rep. 2018; 133: 423-31.
- Centers for Disease Control and Prevention. Drug Overdose Death Data https://www.cdc.gov/drugoverdose/data/statedeaths.html. Centers for Disease Control and Prevention. p. 2018; 2017; [cited Jul 9 2018]. Available from: https://www.cdc.gov/drugoverdose/data/statedeaths.html.
- CDC. Drug overdose deaths in the U.S. Top 100,000 annually. In: CDC, editor. Atlanta: Centers for Disease Control; 2021.
- Bailey GL, Herman DS, Stein MD. Perceived relapse risk and desire for medication assisted treatment among persons seeking inpatient opiate detoxification. J Subst Abuse Treat. 2013; 45: 302-5.
- Sigmon SC, Bisaga A, Nunes EV, O’Connor PG, Kosten T, et al. Opioid detoxification and naltrexone induction strategies: recommendations for clinical practice. Am J Drug Alcohol Abuse. 2012; 38: 187-99.
- Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies — tackling the opioid-overdose epidemic. N Engl J Med. 2014; 370: 2063-6.
- Beetham T, Saloner B, Wakeman SE, Gaye M, Barnett ML. Access to office-based buprenorphine treatment in areas with high rates of opioid-related mortality: an audit study. Ann Intern Med. 2019; 171: 1-9.
- Bell J, Strang J. Medication treatment of opioid use disorder. Biol Psychiatry. 2020; 87: 82-8.
- Lagisetty PA, Bohnert A. Role of an accurate treatment locator and cash-only practices in access to buprenorphine for opioid use disorders. Ann Intern Med. 2019; 171: 58-9.
- Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009; 3: CD002209.
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, et al. Injectable extended-release naltrexone for opioid dependence: A double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011; 377: 1506-13.
- Lee JD, Friedmann PD, Kinlock TW, Nunes EV, Boney TY, et al. Extended-release naltrexone to prevent opioid relapse in criminal justice offenders. N Engl J Med. 2016; 374: 1232-42.
- Tanum L, Solli KK, Latif ZE, Benth JŠ, Opheim A, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: A randomized clinical noninferiority trial. JAMA Psychiatry. 2017; 74: 1197-205.
- Strang J, McCambridge J, Best D, Beswick T, Bearn J, et al. Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ. 2003; 326: 959-60.
- Sullivan M, Bisaga A, Pavlicova M, Choi CJ, Mishlen K, et al. Long-acting injectable naltrexone induction: A randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017; 174: 459-67.
- Fishman M, Tirado C, Alam DS, Gullo K, Clinch T, et al. Safety and efficacy of lofexidine for medically managed opioid withdrawal: A randomized controlled clinical trial. J Addict Med. 2019; 13: 169-76.
- Alam D, Tirado C, Pirner M, Clinch T. Efficacy of lofexidine for mitigating opioid withdrawal symptoms: results from two randomized, placebo-controlled trials. J Drug Assess. 2020; 9: 13-9.
- Gorodetzky CW, Walsh SL, Martin PR, Saxon AJ, Gullo KL, et al. A phase Iii, randomized, multi-center, double blind, placebo controlled study of safety and efficacy of lofexidine for relief of symptoms in individuals undergoing inpatient opioid withdrawal. Drug Alcohol Depend. 2017; 176: 79-88.
- Nestler EJ, Lüscher C. The molecular basis of drug addiction: linking epigenetic to synaptic and circuit mechanisms. Neuron. 2019; 102: 48-59.
- Kalivas PW, Nakamura M. Neural systems for behavioral activation and reward. Curr Opin Neurobiol. 1999; 9: 223-7.
- Kalivas PW, Duffy P. Similar effects of daily cocaine and stress on mesocorticolimbic dopamine neurotransmission in the rat. Biol Psychiatry. 1989; 25: 913-28.
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003; 37: 577-82.
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996; 23: 28-38.
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: Ii. Clinical studies. Synapse. 1996; 23: 39-51.
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, et al. Elevated Csf corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997; 154: 624-9.
- Baker DG, Ekhator NN, Kasckow JW, Dashevsky B, Horn PS, et al. Higher levels of basal serial Csf cortisol in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 2005; 162: 992-4.
- Vythilingam M, Anderson GM, Owens MJ, Halaszynski TM, Bremner JD, et al. Cerebrospinal fluid corticotropin-releasing hormone in healthy humans—effects of yohimbine and naloxone. J Clin Endocrinol Metab. 2000; 85: 4138-45.
- Walter M, Gerber H, Kuhl HC, Schmid O, Joechle W, et al. Acute effects of intravenous heroin on the hypothalamic-pituitary-adrenal axis response: A controlled trial. J Clin Psychopharmacol. 2013; 33: 193-8.
- Kosten TR, Krystal J. Biological mechanisms in post traumatic stress disorder: relevance for substance abuse. Recent Dev Alcohol. 1988; 6: 49-68.
- Bremner JD, Southwick SM, Darnell A, Charney DS. Chronic Ptsd in Vietnam combat veterans: course of illness and substance abuse. Am J Psychiatry. 1996; 153: 369-75.
- Wachholtz A, Foster S, Cheatle M. Psychophysiology of pain and opioid use: implications for managing pain in patients with an opioid use disorder. Drug Alcohol Depend. 2015; 146: 1-6.
- Back SE, Gros DF, McCauley JL, Flanagan JC, Cox E, et al. Laboratory-induced cue reactivity among individuals with prescription opioid dependence. Addict Behav. 2014; 39: 1217-23.
- Back SE, Gros DF, Price M, LaRowe S, Flanagan J, et al. Laboratory-induced stress and craving among individuals with prescription opioid dependence. Drug Alcohol Depend. 2015; 155: 60-7.
- Gilmore AK, Guille C, Baker NL, Brady KT, Hahn CK, et al. Gender differences in subjective stress and neuroendocrine response to a stress task among individuals with opioid dependence: A pilot study. Addict Behav. 2019; 92: 148-54.
- Coffey SF, Saladin ME, Drobes DJ, Brady KT, Dansky BS, et al. Trauma and substance cue reactivity in individuals with comorbid posttraumatic stress disorder and cocaine or alcohol dependence. Drug Alcohol Depend. 2002; 65: 115-27.
- Gros DF, Szafranski DD, Brady KT, Back SE. Relations between pain, Ptsd symptoms, and substance use in veterans. Psychiatry. 2015; 78: 277-87.
- Charney DS, Heninger GR, Kleber HD. The combined use of clonidine and naltrexone as a rapid, safe, and effective treatment of abrupt withdrawal from methadone. Am J Psychiatry. 1986; 143: 831-7.
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, et al. Injectable, sustained-release naltrexone for the treatment of opioid dependence: A randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006; 63: 210-8.
- Lott DC. Extended release naltrexone: good but not a panacea. Lancet. 2018; 391: 283-4.
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003; 111: 1444-51.
- Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog Brain Res. 1990; 85: 367-402.
- Brewer JA, Bowen S, Smith JT, Marlatt GA, Potenza MN. Mindfulness-based treatments for co-occurring depression and substance use disorders: what can we learn from the brain? Addiction. 2010; 105: 1698-706.
- Stein EA. Fmri: A new tool for the in vivo localization of drug actions in the brain. J Anal Toxicol. 2001; 25: 419-24.
- Howell LL, Murnane KS. Nonhuman primate positron emission tomography neuroimaging in drug abuse research. J Pharmacol Exp Ther. 2011; 337: 324-34.
- Murnane KS, Howell LL. Neuroimaging and drug taking in primates. Psychopharmacology. 2011; 216: 153-71.
- Cabrera EA, Wiers CE, Lindgren E, Miller G, Volkow ND, et al. Neuroimaging the effectiveness of substance use disorder treatments. J Neuroimmune Pharmacol. 2016; 11: 408-33.
- Kreek MJ. Molecular and cellular neurobiology and pathophysiology of opiate addiction. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: the fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins. 2002; 1491-506.
- Childress AR, McLellan AT, O’Brien CP. Conditioned responses in a methadone population: A comparison of laboratory, clinic, and natural settings. J Subst Abuse Treat. 1986; 3: 173-9.
- LeDoux JE. The emotional brain: the mysterious underpinnings of emotional life. New York: Simon & Schuster; 1996.
- Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem. 2002; 9: 402-7.
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992; 15: 353-75.
- LeDoux JE. Emotional memory: In search of systems and synapses. Ann N Y Acad Sci. 1993; 702: 149-57.
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, et al. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005; 35: 791-806.
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci. 2003; 985: 389-410.
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, et al. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005; 57: 464-73.
- Armony JL, Corbo V, Clément MH, Brunet A. Amygdala response in patients with acute Ptsd to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005; 162: 1961-3.
- Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, et al. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an Fmri study. Hum Brain Mapp. 2008; 29: 517-23.
- Kemp AH, Felmingham K, Das P, Hughes G, Peduto AS, et al. Influence of comorbid depression on fear in posttraumatic stress disorder: an Fmri study. Psychiatry Res. 2007; 155: 265-9.
- Kemp AH, Felmingham KL, Falconer E, Liddell BJ, Bryant RA, et al. Heterogeneity of non-conscious fear perception in posttraumatic stress disorder as a function of physiological arousal: an Fmri study. Psychiatry Res. 2009; 174: 158-61.
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional Mri study. Biol Psychiatry. 2000; 47: 769-76.
- Felmingham KL, Williams LM, Kemp AH, Rennie C, Gordon E, et al. Anterior cingulate activity to salient stimuli is modulated by autonomic arousal in posttraumatic stress disorder. Psychiatry Res. 2009; 173: 59-62.
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, et al. Exaggerated and disconnected insular-amygdalar Blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol Psychiatry. 2010; 68: 433-41.
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005; 62: 273-81.
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus coeruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983; 63: 844-914.
- Rasmussen K, Aghajanian GK. Withdrawal-induced activation of locus coeruleus neurons in opiate-dependent rats: attenuation by lesion of the nucleus paragigantocellularis. Brain Res. 1989;505(2):346-50.
- Korf J, Bunney BS, Aghajanian GK. Noradrenergic neurons: morphine inhibition of spontaneous activity. Eur J Pharmacol. 1974; 25: 165-9.
- Nestler EJ. Under siege: the brain on opiates. Neuron. 1996; 16: 897-900.
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997; 278: 58-63.
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009; 56: 3-8.
- London ED, Broussolle EPM, Links JM, Wong DF, Cascella NG, et al. Morphine-induced metabolic changes in human brain: studies with positron emission tomography and [fluorine-18]fluorodeoxyglucose. Arch Gen Psychiatry. 1990; 47: 73-81.
- Liu J, Liang J, Qin W, Tian J, Yuan K, et al. Dysfunctional connectivity patterns in chronic heroin users: an Fmri study. Neurosci Lett. 2009; 460: 72-7.
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, et al. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002; 59: 1162-72.
- Liu J, Qin W, Yuan K, Li J, Wang W, et al. Interaction between dysfunctional connectivity at rest and heroin cues-induced brain responses in male abstinent heroin-dependent individuals. PLOS ONE. 2011; 6: e23098.
- Schmidt A, Walter M, Gerber H, Seifritz E, Brenneisen R, et al. Normalizing effect of heroin maintenance treatment on stress-induced brain connectivity. Brain. 2015; 138: 217-28.
- Shi J, Zhao LY, Copersino ML, Fang YX, Chen Y, et al. Pet imaging of dopamine transporter and drug craving during methadone maintenance treatment and after prolonged abstinence in heroin users. Eur J Pharmacol. 2008; 579: 160-6.
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007; 64: 1575-9.
- Anda RF, Felitti VJ, Walker J, Whitfield C, Bremner JD, et al. The enduring effects of childhood abuse and related experiences in childhood: A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006; 256: 174-86.
- Grice DE, Brady KT, Dustan LR, Malcolm R, Kilpatrick DG. Sexual and physical assault history and post traumatic stress disorder in substance dependent individuals. Am J Addict. 1995; 4: 297-305.
- Farrugia PL, Mills KL, Barrett E, Back SE, Teesson M, et al. Childhood trauma among individuals with co-morbid substance use and post traumatic stress disorder. Ment Health Subst Use. 2011; 4: 314-26.
- Lawson KM, Back SE, Hartwell KJ, Moran-Santa Maria M, Brady KT. A comparison of trauma profiles among individuals with prescription opioid, nicotine or cocaine dependence. Am J Addict. 2013; 22: 127-31.
- Ladwig GB, Andersen MD. Substance abuse in women: relationship between chemical dependency in women and past reports of physical and sexual abuse. Int J Addict. 1989; 24: 739-54.
- Oswald LM, Dunn KE, Seminowicz DA, Storr CL. Early life stress and risks for opioid misuse: review of data supporting neurobiological underpinnings. J Pers Med. 2021; 11: 315.
- Dunn KE, Turner GM, Oswald LM. Effects of early life trauma on risks for adult opioid use disorder are mediated by stress and occur independent of depression and anxiety. J Addict Med. 2022; 16: 709-15.
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, et al. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003; 111: 564-72.
- Afifi TO, Henriksen CA, Asmundson GJG, Sareen J. Childhood maltreatment and substance use disorders among men and women in a nationally representative sample. Can J Psychiatry. 2012; 57: 677-86.
- Brady KT, Killeen T, Saladln ME, Dansky B, Becker S. Comorbid substance abuse and post traumatic stress disorder: characteristics of women in treatment. Am J Addict. 1994; 3: 160-4.
- McCauley JL, Killeen T, Gros DF, Brady KT, Back SE. Posttraumatic stress disorder and co-occurring substance use disorders: advances in assessment and treatment. Clin Psychol (New York). 2012; 19: 1-27.
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008; 1141: 105-30.
- Welsh JW, Knight JR, Hou SS, Malowney M, Schram P, et al. Association between substance use diagnoses and psychiatric disorders in an adolescent and young adult clinic-based population. J Adolesc Health. 2017; 60: 648-52.
- Fareed A, Eilender P, Haber M, Bremner JD, Whitfield N, et al. Comorbid posttraumatic stress disorder and opiate addiction: A literature review. J Addict Dis. 2013; 32: 168-79.
- Chilcoat HD, Breslau N. Posttraumatic stress disorder and drug disorders: testing causal pathways. Arch Gen Psychiatry. 1998; 55: 913-7.
- Nazarian D, Kimerling R, Frayne SM. Posttraumatic stress disorder, substance use disorders, and medical comorbidity among returning U.S. veterans. J Trauma Stress. 2012; 25: 220-5.
- Ouimette P, Goodwin E, Brown PJ. Health and well being of substance use disorder patients with and without posttraumatic stress disorder. Addict Behav. 2006; 31: 1415-23.
- Saladin ME, Drobes DJ, Coffey SF, Dansky BS, Brady KT, et al. Ptsd symptom severity as a predictor of cue-elicited drug craving in victims of violent crime. Addict Behav. 2003; 28: 1611-29.
- Back SE, Lawson KM, Singleton LM, Brady KT. Characteristics and correlates of men and women with prescription opioid dependence. Addict Behav. 2011; 36: 829-34.
- Bikson M, Unal G, Brunoni A, Loo C. What psychiatrists need to know about transcranial direct current stimulation. Psychiatric Times. 2017; 1-3.
- Adair D, Truong D, Esmaeilpour Z, Gebodh N, Borges H, et al. Electrical stimulation of cranial nerves in cognition and disease. Brain Stimul. 2020; 13: 717-50.
- Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. 2016; 9: 641-61.
- Tortella G, Casati R, Aparicio LVM, Mantovani A, Senço N, et al. Transcranial direct current stimulation in psychiatric disorders. World J Psychiatry. 2015; 5: 88-102.
- Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, et al. A technical guide to Tdcs, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016; 127: 1031-48.
- Schachter SC, Saper CB. Vagus nerve stimulation. Epilepsia. 1998; 39: 677-86.
- Bremner JD, Rapaport MH. Vagus nerve stimulation: back to the future. Am J Psychiatry. 2017; 174: 609-10.
- Bremner JD, Gurel NZ, Wittbrodt MT, Shandhi MH, Rapaport MH, et al. Application of non-invasive vagal nerve stimulation to stress-related psychiatric disorders. J Pers Med. 2020; 10: 119.
- Marangell LB, Rush AJ, George MS, Sackeim HA, Johnson CR, et al. Vagus nerve stimulation (Vns) for major depressive episodes: longer-term outcome. Biol Psychiatry. 2002; 51: 280-7.
- Sackeim HA, Rush AJ, George MS, Marangell LB, Husain MM, et al. Vagus nerve stimulation (Vns) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001; 25: 713-28.
- Busch V, Zeman F, Heckel A, Menne F, Ellrich J, et al. The effect of transcutaneous vagus nerve stimulation on pain perception – an experimental study. Brain Stimul. 2013; 6: 202-9.
- Babygirija R, Sood M, Kannampalli P, Sengupta JN, Miranda A. Percutaneous electrical nerve field stimulation modulates central pain pathways and attenuates post-inflammatory visceral and somatic hyperalgesia in rats. Neuroscience. 2017; 356: 11-21.
- Agorastos A, Boel JA, Heppner PS, Hager T, Moeller-Bertram T, et al. Diminished vagal activity and blunted diurnal variation of heart rate dynamics in posttraumatic stress disorder. Stress. 2013; 16: 300-10.
- George MS, Ward HE, Ninan PT, Pollack M, Nahas Z, et al. A pilot study of vagus nerve stimulation (Vns) for treatment-resistant anxiety disorders. Brain Stimul. 2008; 1: 112-21.
- Barbanti P, Grazzi L, Egeo G, Padovan AM, Liebler E, et al. Non-invasive vagus nerve stimulation for acute treatment of high-frequency and chronic migraine: an open-label study. J Headache Pain. 2015; 16: 61.
- Nesbitt AD, Marin JCA, Tomkins E, Ruttledge MH, Goadsby PJ. Non-invasive vagus nerve stimulation for the treatment of cluster headache: A case series. J Headache Pain. 2013; 14: 1.
- Ben-Menachem E, Revesz D, Simon BJ, Silberstein S. Surgically Implanted and Non-Invasive vagus nerve Stimulation: a review of Efficacy, Safety and Tolerability. Eur J Neurol. 2015; 22: 1260-8.
- Gaul C, Magis D, Liebler EJ, Straube A. Effects of non-invasive vagus nerve stimulation on attack frequency over time and expanded response rates in patients with chronic cluster headache: A post hoc analysis of the randomized, controlled Preva study. J Headache Pain. 2017; 18: 22.
- Ben-Menachem E, Hellström K, Waldton C, Augustinsson LE. Evaluation of refractory epilepsy treated with vagus nerve stimulation for up to 5 years. Neurology. 1999; 52: 1265-7.
- Ben-Menachem E, Mañon-Espaillat R, Ristanovic R, Wilder BJ, Stefan H, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994; 35: 616-26.
- George R, Salinsky M, Kuzniecky R, Rosenfeld W, Bergen D, et al. Vagus nerve stimulation for treatment of partial seizures: 3. Long-term follow-up on the first 67 patients exiting a controlled study. Epilepsia. 1994; 35: 637-43.
- Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, et al. Vagus nerve stimulation therapy for partial-onset seizures: A randomized active-control trial. Neurology. 1998; 51: 48-55.
- Salinsky MC, Uthman BM, Ristanovic RK, Wernicke JF, Tarver WB. Vagus nerve stimulation for the treatment of medically intractable seizures. Results of a 1-year open-extension trial. The vagus nerve stimulation study group. Arch Neurol. 1999; 53: 1176-80.
- The Vagus Nerve Stimulation Study Group. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. The Vagus Nerve Stimulation Study Group. Neurology. 1995; 45: 224-30.
- Berry SM, Broglio K, Bunker M, Jayewardene A, Olin B, et al. A patient-level meta-analysis of studies evaluating vagus nerve stimulation therapy for treatment-resistant depression. Med Devices (Auckl). 2013; 6: 17-35.
- Brunoni AR, Moffa AH, Fregni F, Palm U, Padberg F, et al. Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. Br J Psychiatry. 2016; 208: 522-31.
- Brunoni AR, Moffa AH, Sampaio-Junior B, Borrione L, Moreno ML, et al. Trial of electrical direct-current therapy versus escitalopram for depression. N Engl J Med. 2017; 376: 2523-33.
- Brunoni AR, Valiengo L, Baccaro A, Zanão TA, de Oliveira JF, et al. The sertraline versus electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013; 70: 383-91.
- Dell’Osso B, Oldani L, Palazzo MC, Balossi I, Ciabatti M, et al. Vagus nerve stimulation in treatment-resistant depression: acute and follow-up results of an Italian case series. J ECT. 2013; 29: 41-4.
- George MS, Rush AJ, Marangell LB, Sackeim HA, Brannan SK, et al. A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol Psychiatry. 2005; 58: 364-73.
- George MS, Rush AJ, Sackeim HA, Marangell LB. Vagus nerve stimulation (Vns): utility in neuropsychiatric disorders. Int J Neuropsychopharmacol. 2003; 6: 73-83.
- Rush AJ, George MS, Sackeim HA, Marangell LB, Husain MM, et al. Vagus nerve stimulation (Vns) for treatment-resistant depression: A multicenter study. Biol Psychiatry. 2000; 47: 276-86.
- Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan SK, et al. Vagus nerve stimulation for treatment-resistant depression: A randomized, controlled acute phase trial. Biol Psychiatry. 2005; 58: 347-54.
- Rush AJ, Sackeim HA, Marangell LB, George MS, Brannan SK, et al. Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: A naturalistic study. Biol Psychiatry. 2005; 58: 355-63.
- Sackeim HA, Brannan SK, Rush AJ, George MS, Marangell LB, et al. Durability of antidepressant response to vagus nerve stimulation (Vns). Int J Neuropsychopharmacol. 2007; 10: 817-26.
- Sackeim HA, Keilp JG, Rush AJ, George MS, Marangell LB, et al. The effects of vagus nerve stimulation on cognitive performance in patients with treatment-resistant depression. Neuropsychiatry Neuropsychol Behav Neurol. 2001; 14: 53-62.
- Engineer CT, Engineer ND, Riley JR, Seale JD, Kilgard MP. Pairing speech sounds with vagus nerve stimulation drives stimulus-specific cortical plasticity. Brain Stimul. 2015; 8: 637-44.
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, et al. Reversing pathological neural activity using targeted plasticity. Nature. 2011; 470: 101-4.
- Kim HJ, Shim H-J, Kwak MY, An Y-H, Kim DH, et al. Feasibility and safety of transcutaneous vagus nerve stimulation paired with notched music therapy for the treatment of chronic tinnitus. J Audiol Otol. 2015; 18: 159-67.
- Li TT, Wang ZJ, Yang SB, Zhu JH, Zhang SZ, et al. Transcutaneous electrical stimulation at auricular acupoints innervated by auricular branch of vagus nerve pairing tone for tinnitus: study protocol for a randomized controlled clinical trial. Trials. 2015; 16: 101.
- Liu AF, Zhao FB, Wang J, Lu YF, Tian J, Zhao Y, et al. Effects of vagus nerve stimulation on cognitive functioning in rats with cerebral ischemia reperfusion. J Transl Med. 2016; 14: 1-12.
- Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci. 1999; 2: 94-8.
- Suthana N, Fried I. Deep brain stimulation for enhancement of learning and memory. Neuroimage. 2014; 85: 996-1002.
- Zuo Y, Smith DC, Jensen RA. Vagus nerve stimulation potentiates hippocampal Ltp in freely moving rats. Physiol Behav. 2007; 90: 583-9.
- Brock C, Brock B, Aziz Q, Møller HJ, Pfeiffer Jensen M, et al. Transcutaneous cervical vagal nerve stimulation modulates cardiac vagal tone and tumor necrosis factor-alpha. Neurogastroenterol Motil. 2017; 29: 1-4.
- Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, et al. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul. 2014; 7: 871-7.
- Hammond EJ, Uthman BM, Wilder BJ, Ben-Menachem E, Hamberger A, et al. Neurochemical effects of vagus nerve stimulation in humans. Brain Res. 1992; 583: 300-3.
- Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007; 74: 224-42.
- Weber CS, Thayer JF, Rudat M, Wirtz PH, Zimmermann-Viehoff F, et al. Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine, and immune markers. Eur J Appl Physiol. 2010; 109: 201-11.
- Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, et al. Power spectral analysis of heart rate and arterial pressure variability as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986; 59: 178-93.
- Bansal V, Ryu SY, Lopez N, Allexan S, Krzyzaniak M, et al. Vagal stimulation modulates inflammation through a ghrelin mediated mechanism in traumatic Brain injury. Inflammation. 2012; 35: 214-20.
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000; 405: 458-62.
- Corsi-Zuelli FMDG, Brognara F, Quirino GFDS, Hiroki CH, Fais RS, et al. Neuroimmune interactions in schizophrenia: focus on vagus nerve stimulation and activation of the Alpha-7 nicotinic acetylcholine receptor. Front Immunol. 2017; 8: 618.
- Cunningham JT, Mifflin SW, Gould GG, Frazer A. Induction of C-Fos and delta-Fosb immunoreactivity in rat Brain by vagal nerve stimulation. Neuropsychopharmacology. 2008; 33: 1884-95.
- De Herdt V, Bogaert S, Bracke KR, Raedt R, De Vos M, et al. Effects of vagus nerve stimulation on pro- and anti-inflammatory cytokine induction in patients with refractory epilepsy. J Neuroimmunol. 2009; 214: 104-8.
- Lerman I, Hauger R, Sorkin L, Proudfoot J, Davis B, et al. Noninvasive transcutaneous vagus nerve stimulation decreases whole blood culture-derived cytokines and chemokines: A randomized, blinded, healthy control pilot trial. Neuromodulation. 2016; 19: 283-90.
- Li W, Olshansky B. Inflammatory cytokines and nitric oxide in heart failure and potential modulation by vagus nerve stimulation. Heart Fail Rev. 2011; 16: 137-45.
- Majoie HJM, Rijkers K, Berfelo MW, Hulsman JARJ, Myint A, et al. Vagus nerve stimulation in refractory epilepsy: effects on pro- and anti-inflammatory cytokines in peripheral blood. Neuroimmunomodulation. 2011; 18: 52-6.
- Marin MF, Camprodon JA, Dougherty DD, Milad MR. Device-based brain stimulation to augment fear extinction: implications for Ptsd treatment and beyond. Depress Anxiety. 2014; 31: 269-78.
- McLaughlin KA, Alves S, Sheridan MA. Vagal regulation and internalizing psychopathology among adolescents exposed to childhood adversity. Dev Psychobiol. 2014; 56: 1036-51.
- Noble IJ, Gonzalez IJ, Meruva VB, Callahan KA, Belfort BD, et al. Effects of vagus nerve stimulation on extinction of conditioned fear and post-traumatic stress disorder symptoms. Psychiatry Rats, translator. 2017; 7: 1-8.
- Peña DF, Childs JE, Willett S, Vital A, McIntyre CK, et al. Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Front Behav Neurosci. 2014; 8: 327.
- Peña DF, Engineer ND, McIntyre CK. Rapid remission of conditioned fear expression with extinction training paired with vagus nerve stimulation. Biol Psychiatry. 2013; 73: 1071-7.
- Polak T, Markulin F, Ehlis AC, Langer JBM, Ringel TM, et al. Far field potentials from Brain stem after transcutaneous vagus nerve stimulation: optimization of stimulation and recording parameters. J Neural Transm (Vienna). 2009; 116: 1237-42.
- Childs JE, DeLeon J, Nickel E, Kroener S. Vagus nerve stimulation reduces cocaine seeking and alters plasticity in the extinction network. Learn Mem. 2017; 24: 35-42.
- Perez SM, Carreno FR, Frazer A, Lodge DJ. Vagal nerve stimulation reverses aberrant dopamine system function in the methylazoxymethanol acetate rodent model of schizophrenia. J Neurosci. 2014; 34: 9261-7.
- Brunelin J, Mondino M, Gassab L, Haesebaert F, Gaha L, et al. Examining transcranial direct-current stimulation (Tdcs) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012; 169: 719-24.
- Hasan A, Wolff-Menzler C, Pfeiffer S, Falkai P, Weidinger E, et al. Transcutaneous noninvasive vagus nerve stimulation (Tvns) in the treatment of schizophrenia: A bicentric randomized controlled pilot study. Eur Arch Psychiatry Clin Neurosci. 2015; 265: 589-600.
- D’Urso G, Brunoni AR, Mazzaferro MP, Anastasia A, de Bartolomeis A, et al. Transcranial direct current stimulation for obsessive-compulsive disorder: A randomized, controlled, partial crossover trial. Depress Anxiety. 2016; 33: 1132-40.
- Lamb DG, Porges EC, Lewis GF, Williamson JB. Non-invasive vagal nerve stimulation effects on hyperarousal and autonomic state in patients with posttraumatic stress disorder and history of mild traumatic Brain injury: preliminary evidence. Front Med (Lausanne). 2017; 4: 124.
- Gurel NZ, Mobashir HS, Bremner JD, Vaccarino V, Ladd SL, et al. Toward closed-loop transcutaneous vagus nerve stimulation using peripheral cardiovascular physiological biomarkers: A proof-of-concept study. IEEE Body Sens Netw (BSN). 2018: 78-81.
- Bremner JD, Gurel N, Wittbrodt M, Nye J, Alam Z, et al. Non-invasive vagal nerve stimulation paired with stress exposure in posttraumatic stress disorder (Ptsd). Brain Stimul. 2019; 12: 438.
- Milev RV, Giacobbe P, Kennedy SH, Blumberger DM, Daskalakis ZJ, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical guidelines for the management of adults with major depressive disorder: Section 4. Neurostimulation treatments. Can J Psychiatry. 2016; 61: 561-75.
- Feldman RL, Dunner DL, Muller JS, Stone DA. Medicare patient experience with vagus nerve stimulation for treatment-resistant depression. J Med Econ. 2013; 16: 63-74.
- Gazi AH, Gurel NZ, Richardson KLS, Wittbrodt MT, Shah AJ, et al. Digital cardiovascular biomarker responses to transcutaneous cervical vagus nerve stimulation: state-space modeling, prediction, and simulation. JMIR mHealth uHealth. 2020; 8: e20488.
- Gurel NZ, Gazi AH, Scott KL, Wittbrodt MT, Shah AJ, et al. Timing considerations for noninvasive vagal nerve stimulation in clinical studies. AMIA Annu Symp Proc. 2019; 2019: 1061-70.
- Gurel NZ, Huang M, Wittbrodt MT, Jung H, Ladd SL, et al. Quantifying acute physiological biomarkers of transcutaneous cervical vagal nerve stimulation in the context of psychological stress. Brain Stimul. 2020; 13: 47-59.
- Gurel NZ, Wittbrodt MT, Jung H, Shandhi MMH, Driggers EG, et al. Transcutaneous vagal nerve stimulation reduces sympathetic responses to stress in posttraumatic stress disorder: A double-blind, randomized, sham controlled trial. Neurobiol Stress. 2020; 13: 100264.
- Gurel NZ, Wittbrodt MT, Jung H, Ladd SL, Shah AJ, et al. Automatic detection of target engagement in transcutaneous cervical vagal nerve stimulation for traumatic stress triggers. IEEE J Biomed Health Inform. 2020; 24: 1917-25.
- Gazi AH, Sundararaj S, Harrison AB, Gurel NZ, Wittbrodt MT, et al. Transcutaneous cervical vagus nerve stimulation inhibits the reciprocal of the pulse transit Time’s responses to traumatic stress in posttraumatic stress disorder. Annu Int Conf IEEE Eng Med Biol Soc. 2021; 2021: 1444-7.
- Gazi AH, Sundararaj S, Harrison AB, Gurel NZ, Wittbrodt MT, et al. Transcutaneous cervical vagus nerve stimulation lengthens exhalation in the context of traumatic stress. IEEE EMBS Int Conf Biomed Health Inform. EEE EMBS International Conference on Biomedical and Health Informatics (BHI). 2021; 2021.
- Gazi AH, Wittbrodt MT, Harrison AB, Sundararaj S, Gurel NZ, et al. Robust estimation of respiratory variability uncovers correlates of limbic Brain activity and transcutaneous cervical vagus nerve stimulation in the context of traumatic stress. IEEE Trans Bio Med Eng. 2022; 69: 849-59.
- Bremner JD, Gurel NZ, Jiao Y, Wittbrodt MT, Levantsevych OM, et al. Transcutaneous vagal nerve stimulation blocks stress-induced activation of interleukin-6 and interferon-Γ in posttraumatic stress disorder: A double-blind, randomized, sham-controlled trial. Brain Behav Immun Health. 2020; 9: 100138.
- Gurel NZ, Jiao Y, Wittbrodt MT, Hankus A, Driggers EG, et al. Effect of transcutaneous vagus nerve stimulation on the pituitary adenylate cyclase-activating polypeptide (Pacap) response to stress: A randomized, sham controlled, double blind pilot study. Compr Psychoneurendocrinol. 2020; 4: 100012.
- Wittbrodt MT, Gurel NZ, Nye JA, Shandhi MMH, Gazi AH, et al. Non-invasive cervical vagal nerve stimulation alters Brain activity during traumatic stress in individuals with posttraumatic stress disorder. Psychosom Med. 2021; 83: 969-77.
- Wittbrodt MT, Gurel NZ, Nye JA, Ladd S, Shandhi MMH, et al. Non-invasive vagal nerve stimulation decreases Brain activity during trauma scripts. Brain Stimul. 2020; 13: 1333-48.
- Bremner JD, Wittbrodt MT, Gurel NZ, Shandhi MH, Gazi AH, et al. Transcutaneous cervical vagal nerve stimulation in patients with posttraumatic stress disorder (Ptsd): A pilot study of effects on Ptsd symptoms and interleukin-6 response to stress. J Affect Disord Rep. 2021; 6: 100190.
- Wang Z, Yang XR, Song H, Cao BR, Yin F, et al. Immune function alterations during 12 weeks of abstinence in heroin users. Folia Biol. 2015; 61: 241-6.
- Hammer N, Löffler S, Cakmak YO, Ondruschka B, Planitzer U, et al. Cervical vagus nerve morphometry and vascularity in the context of nerve stimulation - a cadaveric study. Sci Rep. 2018; 8: 7997.
- Levy MN. Sympathetic-parasympathetic interactions in the heart. Circ Res. 1971; 29: 437-45.
- Das UN. Can vagus nerve stimulation halt or ameliorate rheumatoid arthritis and lupus? Lipids Health Dis. 2011; 10: 19.
- Hardy SG. Medullary projections to the vagus nerve and posterolateral hypothalamus. Anat Rec. 1995; 242: 251-8.
- Plantinga L, Bremner JD, Miller AH, Jones DP, Veledar E, et al. Association between posttraumatic stress disorder and inflammation: A twin study. Brain Behav Immun. 2013; 30: 125-32.
- Mourdoukoutas AP, Truong DQ, Adair DK, Simon BJ, Bikson M. High-resolution multi-scale computational model for non-invasive cervical vagus nerve stimulation. Neuromodulation. 2018; 21: 261-8.
- Wen J, Desai NS, Jeffery D, Aygun N, Blitz A. High-resolution isotropic three-dimensional mr imaging of the extraforaminal segments of the cranial nerves. Magn Reson Imaging Clin N Am. 2018; 26: 101-19.
- Hays SA, Rennaker RL, Kilgard MP. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog Brain Res. 2013; 207: 275-99.
- Manta S, Dong J, Debonnel G, Blier P. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J Psychiatry Neurosci. 2009; 34: 272-80.
- Hassert DL, Miyashita T, Williams CL. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav Neurosci. 2004; 118: 79-88.
- Roosevelt RW, Smith DC, Clough RW, Jensen RA, Browning RA. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 2006; 1119: 124-32.
- Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, et al. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp Neurol. 2017; 289: 21-30.
- Manta S, Dong J, Debonnel G, Blier P. Optimization of vagus nerve stimulation parameters using the firing activity of serotonin neurons in the rat dorsal raphe. Eur Neuropsychopharmacol. 2009; 19: 250-5.
- Manta S, El Mansari M, Debonnel G, Blier P. Electrophysiological and neurochemical effects of long-term vagus nerve stimulation on the rat monoaminergic systems. Int J Neuropsychopharmacol. 2013; 16: 459-70.
- McGaugh JL. Peripheral and central adrenergic influences on Brain systems involved in the modulation of memory storage. Ann N Y Acad Sci. 1985; 444: 150-61.
- Dorr AE, Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J Pharmacol Exp Ther. 2006; 318: 890-8.
- Nichols JA, Nichols AR, Smirnakis SM, Engineer ND, Kilgard MP, et al. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience. 2011; 189: 207-14.
- Noble LJ, Meruva VB, Hays SA, Rennaker RL, Kilgard MP, et al. Vagus nerve stimulation promotes generalization of conditioned fear extinction and reduces anxiety in rats. Brain Stimul. 2019; 12: 9-18.
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998; 21: 294-9.
- McGaugh JL. Memory - a century of consolidation. Science. 2000; 287: 248-51.
- Bremner JD, Vythilingam M, Vermetten E, Afzal N, Nazeer A, et al. Effects of dexamethasone on declarative memory function in posttraumatic stress disorder (Ptsd). Psychiatry Res. 2004; 129: 1-10.
- Bremner JD, Vythilingam M, Vermetten E, Anderson G, Newcomer JW, Charney DS. Effects of glucocorticoids on declarative memory function in major depression. Biol Psychiatry. 2004; 55: 811-5.
- Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994; 371: 702-4.
- Southwick SM, Davis M, Horner B, Cahill L, Morgan CA, et al. Relationship of enhanced norepinephrine activity during memory consolidation to enhanced long-term memory in humans. Am J Psychiatry. 2002; 159: 1420-2.
- Flood JF, Smith GE, Morley JE. Modulation of memory processing by cholecystokinin: dependence on the vagus nerve. Science. 1987; 236: 832-4.
- Williams CL, Jensen RA. Effects of vagotomy on leuenkephalin-induced impairments in memory storage. Physiol Behav. 1993; 54: 659-63.
- Clark KB, Krahl SE, Smith DC, Jensen RA. Post-training unilateral vagal stimulation enhances retention performance in the rat. Neurobiol Learn Mem. 1995; 63: 213-6.
- Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, et al. Posttraining electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiol Learn Mem. 1998; 70: 364-73.
- Shen H, Fuchino Y, Miyamoto D, Nomura H, Matsuki N. Vagus nerve stimulation enhances perforant path-Ca3 synaptic transmission via the activation of beta-adrenergic receptors and the locus coeruleus. Int J Neuropsychopharmacol. 2012; 15: 523-30.
- Ura H, Sugaya Y, Ohata H, Takumi I, Sadamoto K, et al. Vagus nerve stimulation induced long-lasting enhancement of synaptic transmission and decreased granule cell discharge in the hippocampal dentate gyrus of urethane-anesthetized rats. Brain Res. 2013; 1492: 63-71.
- Revesz D, Tjernstrom M, Ben-Menachem E, Thorlin T. Effects of vagus nerve stimulation on rat hippocampal progenitor proliferation. Exp Neurol. 2008; 214: 259-65.
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003; 301: 805-9.
- Duman RS. Depression: A case of neuronal life and death? Biol Psychiatry. 2004; 56: 140-5.
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997; 54: 597-606.
- Souza RR, Robertson NM, Pruitt DT, Gonzales PA, Hays SA, et al. Vagus nerve stimulation reverses the extinction impairments in a model of Ptsd with prolonged and repeated trauma. Stress. 2019; 22: 509-20.
- Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev. 2005; 29: 493-500.
- Player MJ, Taylor JL, Weickert CS, Alonzo A, Sachdev PS, et al. Increase in pas-induced neuroplasticity after a treatment course of transcranial direct current stimulation for depression. J Affect Disord. 2014; 167: 140-7.
- Hays SA. Enhancing rehabilitative therapies with vagus nerve stimulation. Neurotherapeutics. 2016; 13: 382-94.
- Hays SA, Ruiz A, Bethea T, Khodaparast N, Carmel JB, et al. Vagus nerve stimulation during rehabilitative training enhances recovery of forelimb function after ischemic stroke in aged rats. Neurobiol Aging. 2016; 43: 111-8.
- Khodaparast N, Kilgard MP, Casavant R, Ruiz A, Qureshi I, et al. Vagus nerve stimulation during rehabilitative training improves forelimb recovery after chronic ischemic stroke in rats. Neurorehab Neural Repair. 2016; 30: 676-84.
- Pruitt DT, Schmid AN, Kim LJ, Abe CM, Trieu JL, et al. Vagus nerve stimulation delivered with motor training enhances recovery of function after traumatic Brain injury. J Neurotrauma. 2016; 33: 871-9.
- Kent KM, Smith ER, Redwood DR, Epstein SE. Electrical stability of acutely ischemic myocardium: influences to heart rate and vagal stimulation. Circulation. 1973; 47: 291-8.
- Hays SA, Khodaparast N, Hulsey DR, Ruiz A, Sloan AM, et al. Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage. Stroke. 2014; 45: 3097-100.
- Li M, Zheng C, Sato T, Kawada T, Sugimachi M, et al. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004; 109: 120-4.
- Myers RW, Pearlman AS, Hyman RM, Goldstein RA, Kent KM, et al. Beneficial effects of vagal stimulation and bradycardia during experimental acute myocardial ischemia. Circulation. 1974; 49: 943-7.
- Sjögren MJ, Hellström PT, Jonsson MA, Runnerstam M, Silander HC, et al. Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer’s disease: A pilot study. J Clin Psychiatry. 2002; 63: 972-80.
- Merrill CA, Jonsson MA, Minthon L, Ejnell H. C-son Silander H, et al. Vagus Nerve Stimulation in Patients with Alzheimer’s Disease: Additional Follow-up Results of a Pilot Study through 1 Year. J Clin Psychiatry. 2006; 67: 1171-8.
- Smith DC, Modglin AA, Roosevelt RW, Neese SL, Jensen RA, et al. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. J Neurotrauma. 2005; 22: 1485-502.
- Smith DC, Tan AA, Duke A, Neese SL, Clough RW, et al. Recovery of Function after vagus nerve Stimulation Initiated 24h after Fluid Percussion Brain Injury. J Neurotrauma. 2006; 23: 1549-60.
- Zubelewicz B, Muc-Wierzgoń M, Harbuz MS, Brodziak A. Central single and chronic administration of morphine stimulates corticosterone and interleukin (Il)-6 in adjuvant-induced arthritis. J Physiol Pharmacol. 2000; 51: 897-906.
- Hosoi T, Okuma Y, Nomura Y. Electrical stimulation of afferent vagus nerve induces IL-1β expression in the Brain and activates hpa axis. Am J Physiol Regul Integr Comp Physiol. 2000; 279: R141-7.
- Watkins LR, Maier SF, Goehler LE. Cytokine-to-Brain communication: a review and analysis of alternative mechanisms. Life Sci. 1995; 57: 1011-26.
- Thrivikraman KV, Zejnelovic F, Bonsall RW, Owens MJ. Neuroendocrine homeostasis after vagus nerve stimulation in rats. Psychoneuroendocrinology. 2013; 38: 1067-77.
- Qu J, Tao XY, Teng P, Zhang Y, Guo CL, et al. Blocking Atp-sensitive potassium channel alleviates morphine tolerance by inhibiting Hsp70-Tlr4-Nlrp3-Mediated neuroinflammation. J Neuroinflammation. 2017; 14: 1-17.
- Houghtling RA, Bayer BM. Rapid elevation of plasma interleukin-6 by morphine is dependent on autonomic stimulation of adrenal gland. J Pharmacol Exp Ther. 2002; 300: 213-9.
- Kapasi AA, Gibbons N, Mattana J, Singhal PC. Morphine stimulates mesangial cell Tnf-alpha and nitrite production. Inflammation. 2000; 24: 463-76.
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007; 21: 901-12.
- Sugama S, Conti B. Interleukin-18 and stress. Brain Res Rev. 2008; 58: 85-95.
- Lima BB, Hammadah M, Wilmot K, Pearce BD, Shah A, et al. Posttraumatic stress disorder is associated with enhanced interleukin-6 response to mental stress in subjects with a recent myocardial infarction. Brain Behav Immun. 2019; 75: 26-33.
- Lashkarizadeh MR, Garshasbi M, Shabani M, Dabiri S, Hadavi H, et al. Impact of opium addiction on levels of pro- and anti-inflammatory cytokines after surgery. Addict Health. 2016; 8: 9-15.
- Dyuizen I, Lamash NE. Histo- and immunocytochemical detection of inducible nos and Tnf-alpha in the locus coeruleus of human opiate addicts. J Chem Neuroanat. 2009; 37: 65-70.
- Nizri E, Brenner T. Modulation of inflammatory pathways by the immune cholinergic system. Amino Acids. 2013; 45: 73-85.
- Pearce BD. Neuroendocrine-immune interactions during viral infections. Adv Virus Res. 2001; 56: 465-509.
- Huston JM, Gallowitsch-Puerta M, Ochani M, Ochani K, Yuan R, et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med. 2007; 35: 2762-8.
- Wang XW, Karki A, Du DY, Zhao XJ, Xiang XY, et al. Plasma levels of high mobility group box 1 increase in patients with posttraumatic stress disorder after severe blunt chest trauma: A prospective cohort study. J Surg Res. 2015; 193: 308-15.
- Olofsson PS, Levine YA, Caravaca A, Chavan SS, Pavlov VA, et al. Single-pulse and unidirectional electrical activation of the cervical vagus nerve reduces tumor necrosis factor in endotoxemia. Bioelectron Med. 2015; 2: 37-42.
- Zhou J, Nagarkatti P, Zhong Y, Ginsberg JP, Singh NP, et al. Dysregulation in microRNA expression is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PLOS ONE. 2014; 9: e94075.
- Bremner JD, Vermetten E. The hippocampus and post-traumatic stress disorders. In: Bartsch T, editor. The clinical neurobiology of the hippocampus: an integrative view. Oxford University Press. 2012; 262-72.
- Das S, Basu A. Inflammation: A new candidate in modulating adult neurogenesis. J Neurosci Res. 2008; 86: 1199-208.
- Myint AM. Kynurenines: from the perspective of major psychiatric disorders. FEBS Journal. 2012; 279: 1375-85.
- Klinkenberg S, van den Borne CJ, Aalbers MW, Verschuure P, Kessels AG, et al. The effects of vagus nerve stimulation on tryptophan metabolites in children with intractable epilepsy. Epilepsy Behav. 2014; 37: 133-8.
- Chakravarthy K, Chaudhry H, Williams K, Christo PJ. Review of the uses of vagal nerve stimulation in chronic pain management. Curr Pain Headache Rep. 2015; 19: 54.
- Bohning DE, Lomarev MP, Denslow S, Nahas Z, Shastri A, et al. Vagus nerve stimulation (Vns) synchronized bold-Fmri. Radiology. 2001; 36: 470-9.
- Chae JH, Nahas Z, Lomarev M, Denslow S, Lorberbaum JP, et al. A review of functional neuroimaging studies of vagus nerve stimulation (Vns). J Psychiatr Res. 2003; 37: 443-55.
- Yakunina N, Kim SS, Nam EC. Optimization of transcutaneous vagus nerve stimulation using functional Mri. Neuromodulation. 2017; 20: 290-300.
- Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology. 2002; 59: S3-14.
- Henry TR, Bakay RA, Votaw JR, Pennell PB, Epstein CM, et al. Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia. 1998; 39: 983-90.
- Conway CR, Sheline YI, Chibnall JT, Bucholz RD, Price JL, et al. Brain blood-flow change with acute vagus nerve stimulation in treatment-refractory major depressive disorder. Brain Stimul. 2012; 5: 163-71.
- Fang J, Egorova N, Rong P, Liu J, Hong Y, et al. Early cortical biomarkers of longitudinal transcutaneous vagus nerve stimulation treatment success in depression. NeuroImage Clin. 2017; 14: 105-11.
- Liu J, Fang J, Wang Z, Rong P, Hong Y, et al. Transcutaneous vagus nerve stimulation modulates amygdala functional connectivity in patients with depression. J Affect Disord. 2016; 205: 319-26.
- Lomarev M, Denslow S, Nahas Z, Chae JH, George MS, et al. Vagus Nerve Stimulation (Vns): synchronized Bold Fmri Suggests That Vns in Depressed Adults Has Frequency and/or Dose Dependent Effects at Rest and During a Simple Task. J Psychiatr Res. 2002; 36: 219-27.
- Van Laere K, Vonck K, Boon P, Versijpt J, et al. Perfusion Spect changes after acute and chronic vagus nerve stimulation in relation to prestimulus condition and long-term efficacy. J Nucl Med. 2002; 43: 733-44.
- Sperling W, Reulbach U, Bleich S, Padberg F, Kornhuber J, et al. Cardiac effects of vagus nerve stimulation in patients with major depression. Pharmacopsychiatry. 2010; 43: 7-11.
- Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: Fmri evidence in humans. Brain Stimul. 2015; 8: 624-36.
- Frangos E, Komisaruk BR. Access to vagal projections via cutaneous electrical stimulation of the neck: Fmri evidence in healthy humans. Brain Stimul. 2017; 10: 19-27.
- Bremner JD, Wittbrodt MT, Gurel NZ, Nye J, Alam A, Vaccarino V, et al. Brain correlates of non-invasive vagal nerve stimulation in stress. NYC Neuromodulation/NANS Conference; Aug 24 2018; New York; 2018; 14.
- Zagorski N. Fda clears way for stimulation device to treat opioid withdrawal symptoms. Psychiatr News. 2018; 2017: 1.
- Miranda A, Taca A. Neuromodulation with percutaneous electrical nerve field stimulation is associated with reduction in signs and symptoms of opioid withdrawal: A multisite, retrospective assessment. Am J Drug Alcohol Abuse. 2018; 44: 56-63.
- Badran BW, Jenkins DD, Cook D, Thompson S, Dancy M, et al. Transcutaneous auricular vagus nerve stimulation-paired rehabilitation for oromotor feeding problems in newborns: an open-label pilot study. Front Hum Neurosci. 2020; 14: 77.
- Jenkins DD, Khodaparast N, O’Leary GH, Washburn SN, Covalin A, et al. Transcutaneous auricular Neurostimulation (Tan): A novel adjuvant treatment in neonatal opioid withdrawal syndrome. Front Hum Neurosci. 2021; 15: 648556.
- Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y. Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology. 2016; 41: 335-56.
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001; 158: 343-59.
- Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to Drug-Taking. J Psychiatry Neurosci. 2000; 25: 125-36.
- Wolpe J, Lazarus AA. Behavioral therapy techniques. New York: Pergamon Press; 1966.
- Inan OT, Baran Pouyan MB, Javaid AQ, Dowling S, Etemadi M, et al. Novel wearable seismocardiography and machine learning algorithms can assess clinical status of heart failure patients. Circ Heart Fail. 2018; 11: e004313.
- Gazi AH, Harrison AB, Lambert TP, Obideen M, Alavi P, et al. Transcutaneous cervical vagus nerve stimulation reduces behavioral and physiological manifestations of withdrawal in patients with opioid use disorder: A double-blind, randomized, sham-controlled trial. Brain Stimul. 2022; 15: 1206-14.
- Gazi AH, Harrison AB, Lambert TP, Nawar A, Obideen M, et al. Pain is reduced by transcutaneous cervical vagus nerve stimulation and correlated with cardiorespiratory variability measures in the context of opioid withdrawal. Front Pain Res (Lausanne). 2022; 3: 1031368.
- Gazi AH, Harrison AB, Lambert TP, Obideen M, Welsh JW, et al. Transcutaneous cervical vagus nerve stimulation reduces respiratory variability in the context of opioid withdrawal. IEEE EMBS Int Conf Biomed Health Inform IEEE-EMBS International Conference on Biomedical and Health Informatics (BHI). 2022; 2022.
- Lambert TP, Gazi AH, Harrison AB, Gharehbaghi S, Chan M, et al. Leveraging accelerometry as a prognostic indicator for increase in opioid withdrawal symptoms. Biosensors. 2022; 12: 924.
- Cicero TJ, Ellis MS. The prescription opioid epidemic: a review of qualitative studies on the progression from initial use to abuse. Dial Clin Neurosci. 2017; 19: 259-69.
- Kosten TR, Baxter LE. Review article: effective management of opioid withdrawal symptoms: A gateway to opioid dependence treatment.36. Am J Addict. 2019; 28: 55-62.
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010; 35: 217-38.
- Pergolizzi JV, Raffa RB, Rosenblatt MH. Opioid withdrawal symptoms, a consequence of chronic opioid use and opioid use disorder: current understanding and approaches to management. J Clin Pharm Ther. 2020; 45: 892-903.
- Rigg KK, Ibañez GE. Motivations for non-medical prescription drug use: A mixed methods analysis. J Subst Abuse Treat. 2010; 39: 236-47.
- Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug Alcohol Depend. 2001; 63: 139-46.
- Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use — United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017; 66: 265-9.
- Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Phys. 2011; 14: 145-61.
- Bommersbach T, Ross DA, De Aquino JP. Perpetual hunger: the neurobiological consequences of long-term opioid use. Biol Psychiatry. 2020; 87: e1-3.
- Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016; 374: 1253-63.
- Dennis BB, Bawor M, Naji L, Chan CK, Varenbut J, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: A systematic review and meta-analysis. Subst Abuse. 2015; 9: 59-80.
- Koob GF. Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry. 2020; 87: 44-53.
- Campanella C, Bremner JD. Neuroimaging of Ptsd. In: Bremner JD, editor. Posttraumatic stress disorder: from neurobiology to treatment. Hoboken, NJ: Wiley-Blackwell. 2016; 291-320.
- Bremner JD, Wittbrodt MT. Stress, the brain, and trauma spectrum disorders. Int Rev Neurobiol. 2020; 152: 1-22.
- Glick G, Braunwald E. Relative roles of the sympathetic and parasympathetic nervous systems in the reflex control of heart rate. Circ Res. 1965; 16: 363-75.
- Southwick SM, Bremner JD, Rasmusson A, Morgan CA, Arnsten A, et al. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999; 46: 1192-204.
- Bremner JD, Innis RB, Ng CK, Staib L, Duncan J, et al. Pet measurement of cerebral metabolic correlates of yohimbine administration in posttraumatic stress disorder. Arch Gen Psychiatry. 1997; 54: 246-54.
- Redgrave J, Day D, Leung H, Laud PJ, Ali A, et al. Safety and tolerability of transcutaneous vagus nerve stimulation in humans: A systematic review. Brain Stimul. 2018; 11: 1225-38.