
Research Article
Austin J Dent. 2016; 3(3): 1037.
Sodium Hypochlorite as a Deproteinizing Agent Optimize Orthodontic Brackets Adhesion using Resin Modified Glass Ionomer Cement
Ayman E1, Amera A2* and Khursheed AM1
1Orthadontic Unit, School of Dental Sciences, University of Science Malaysia, Malaysia
2Department of Oral and Maxillofacial Surgery, University of Anbar, Iraq
*Corresponding author: Alkaisi Amera, Department of Oral and Maxillofacial Surgery, University of Anbar, Iraq
Received: May 21, 2016; Accepted: July 09, 2016; Published: July 12, 2016
Abstract
Aim: The main aim of this study was to test the effect of deproteinization of human dental enamel surfaces, with 5.25% sodium hypochlorite (NaOCl) before etching on orthodontic bracket shear bond strength (SBS) of Resin Modified Glass Ionomer Cement (RMGI) adhesive system.
Materials and Methods: Sixty extracted human premolar teeth were randomly divided into two groups. Group1as an experimental and group II control, with 30 teeth each. Both groups; brackets were bonded to the teeth using, Fuji Ortho LC. The buccal surface of the premolars in the experimental group was deproteinized with 5.25% NaOCl before acid etching and orthodontic brackets were bonded with RMGI. The same protocol was used in the control group except NaOCl was not applied. The debonding force (SBS) was measured using Instron machine and the residual adhesive remain on the tooth surface was scored as well enamel roughness was measured using profilometry. Independent t test was used to determine whether there is a significant difference in SBS and the adhesive remnant index (ARI) scores between the 2 groups. Kurskal-Wallis test was used to test for Ra, Rq and Rt data analysis.
Results: The mean SBS for Fuji Ortho LC with NaOCl, was 17 (±5.37) MPa; and for Fuji Ortho LC without NaOCl, 13.86 (±4.41) MPa, the difference between the 2 groups was significant P =0.029. The mean (SD) adhesive remnant index scores for group 1 & 2 were 3.97 (±.718) and 2.90 (±.712) respectively with significant difference between the 2 groups P > 0.001..No significant difference was found in enamel roughness between cleanup methods P for Ra, Rq and Rt was = 0.340 , 0.483 and 0.280 respectively.
Conclusion: It was concluded that enamel treatment with NaOCl increase bonding strength of brackets bonded with RMGIC, and was statistically significant when compared to the untreated group.
Keywords: Deproteinization; Brackets; RMGI; SBS; ARI
Introduction
The orthodontic communities have strived in the past few years to obtain and use materials and techniques that increase the bond strength between orthodontic brackets and the enamel surface of the teeth. As the attachments have to be intentionally removed upon completion of the treatment, excessive bond strength may cause unwanted damage to enamel surfaces. An acceptable range of bond strength should be sufficiently high to minimize bracket. Debonding complications and bracket debonding by the clinician should be simple, clean, and harmless to the bond restoration. Resin Modified Glass Ionomer Cement (RMGIC), poses the ability to bond in the presence of saliva and blood which can be a very good bonding agent for orthodontic attachments especially in the areas of the mouth that are difficult to access. In addition, their fluoride releasing property makes them an ideal bonding agent for patients with poor oral hygiene [1], however; their immediate bond strength is found to be too low for immediate ligation of the initial wire. Bishara, et al. [2] concluded that RMGIs have significantly lower initial bond strength, but increases more than 20-fold within 24 hrs. In comparison, composite adhesive has a significantly higher initial bond strength that doubled within 24 hrs [3,4]. The low initial bond strength of Fuji Ortho LC necessitates a second appointment for placing the arch wire; which means an increase in the total number of appointments made during the treatment and makes time management more difficult for the orthodontist [1]. Regardless of the bonding technique used to attach orthodontic brackets to the teeth, preparing the enamel surface properly to acquire a good and stable bond is necessary, proper preparation usually requires the removal of the enamel pellicle and the creation of irregularities in the surface prior to bonding, this process is called enamel conditioning [5]. Enamel conditioning is performed using one of the two techniques, the first one; is acid etching, in which an acid gel is utilized, resulting in a microetching and the second is sandblasting, in which air abrasion methods are used, resulting in a macroetching [5]. The introduction of the acid etching technique by Buonocore [6] is a milestone in dentistry, this concept is based on the acid dissolution of the enamel tooth surface resulting in the formation of micro porosities in the surface that are used to achieve a micromechanical bond. Since then, a major modifications and enhancements have ensued, including the use of decreased concentrations of phosphoric acid (80%) to 37% orthophosphoric acid and a reduction in the application time from 60 s to 15 s [6,7]. With the use of 37% phosphoric acid, 15-second treatment is recommended for the anterior teeth and premolars [8]. During routine etching with phosphoric acid, 10 µm to 50 µm enamel is removed from the surface, whereas rough surface porosities up to 10 µm to 200 µm deep are created [9], as well Fjeld and øgaard [10] reported that acid etching causes an enamel loss ranging from 3 µm to 10 µm. A non-invasive technique successfully employed in endodontic the uses of NaOCl as an irrigating solution to disinfect and remove debris and organic materials from the canals [11,12] can be used as a deproteinizing agent. It is a possible strategy to optimize adhesion by removing organic elements of both the enamel structure and the acquired pellicle before acid etching,
Espinosa, et al. [13] suggested that the use of 5.25% NaOCl as a deproteinizing agent prior to acid etching increases bond strength because organic elements are removed well. A universal testing machine (Instron) and a digital dynamometer are used to evaluate the shear bond strength (SBS) in vivo and in vitro of metallic brackets bonded to human teeth with light-cured (LC) bonding materials, It is found that tests performed by a universal testing machine resulted in larger bond strengths than those performed by a digital dynamometer in vivo and in vitro [14]. The debonding force is applied to the junction of the attachment and adhesive interfaces; this method comes closest to applying a true shear force, which never occurs clinically [15]. Adhesive remnant should be entirely removed from the tooth surface after orthodontic attachment removal. However, complete removal of the entire adhesive remnants is not easily achieved because of the colour similarity between the adhesives and enamel, however many patients may be left with unsatisfactory incomplete resin removal [16]. A wide variety of instruments and procedures are used for adhesive removal [17], include manual removal using a scalar or a pair of band-removing pliers [18], various shapes of tungsten carbide burs (TCBs) with low or high speed hand pieces [19, 20], Sof-Lex discs [21], and special composite finishing systems with zirconia paste or slurry pumice as well as ultrasonic applications [22]. Carbon dioxide laser application have also been promising [23], and the Nd:YAG laser has demonstrated potent structural degradation of the composite, suggesting that it could be used as an adjunct to the removal of residual resin [24]. Air powder abrasive systems have also been suggested for removing residual adhesive [25], but the need for rubber dam and protective mask/eye wear is an impractical aspect of this technique [26]. All these techniques produce various degrees of polish, and some introduce abrasion with significant loss of enamel, moreover, they may have adverse effects on the pulp tissues if not dissipated with an appropriate coolant. Approximately 10% of enamel is lost because of acid etching, bracket removal, and cleanup after debonding [27]. This study was aimed to determine the effect of NaOCl application before acid-etching on the shear bond strength of orthodontic brackets bonded to the teeth using resin-modified glass ionomer cement.
Materials and Methods
The experimental procedures were approved by the Research Ethics Committee, University Science Malaysia number: [11:35:30 PM] (FWA Reg. No: 00007718; IRB Reg. No: 00004494). Sixty human premolars, extracted for orthodontic reasons, were collected, the soft tissues removed and the teeth were stored in a distilled water at room temperature (7 days) until they were ready for use. The teeth were randomly divided into 2 different enamel treatment groups with 30 teeth each. Group I: experimental, was treated with sodium hypochlorite 5.25% prior to 37% Phosphoric acid etching using RMGI as an adhesive material and group II: control using 37% Phosphoric acid and RMGI only. Premolars with normal crown shape without any deformity and caries free crowns were included. Teeth which have restorations, cracks, and history of bracket bonding were excluded from the study. The roots of the teeth were embedded in acrylic base frame to make blocks measuring (20 x 20 x 40 mm) for ease of manipulation and testing purposes. Standard orthodontic premolar 0.018 metal brackets (Gemini, 3M Unitek, Monrovia, CA), with a 100-gauge mesh were used in this study.
Laboratory procedures
The buccal surfaces of the premolars were cleaned with a non fluoridated prophylaxis paste and rubber prophylactic cups for 10 seconds, rinsed and dried for both groups.
Group I (NaOCl + acid etching): Each tooth, enamel was deproteinized with 5.25% NaOCl for 1 minute using a micro brush, followed by rinsing, drying, and acid etching with 37% phosphoric acid for 30 seconds. Subsequently, the acid was rinsed off and the enamel dried, RMGI was mixed according to the manufacturer instructions and placed on the bracket mesh covering the entire base of the bracket without bubbles or voids applied to the tooth using force sufficient to produce a flash of excess adhesive around the bracket to ensure a uniform thickness of the adhesive. The excess adhesive was removed with a sharp scalar, and the bracket light cured for ten seconds on each side. A Bluedent smart LED curing light (Plovdiv, bulgaria) was used in all bonding procedures during 40 seconds (10 seconds for each mesial, distal, occlusal, and gingival margins) at emitted wave length of 430-490 nm, as maintaining a distance of 1 mm from the bracket base.
Group II (Acid – Etched group): Same procedures for group 1 were followed except no NaOCl was applied before etching as a control group.
Evaluations
Shear bond strength (SBS): After bracket bonding, the teeth were stored in distilled water for 24 hours at room temperature until they were submitted to the shear test. A Universal Test Machine (Instrone model no.8874, England) was used for bracket debonding, the force applied using a flat-end steel rod at the bracket-tooth interface, measured at a crosshead speed of 1.0 mm / min with the tooth aligned so that the applied force perpendicular to the bracket (Figure 1). Each test was recorded in mega Pascal (MPa) by a computer contact to the machine and the samples were restored in distilled water.
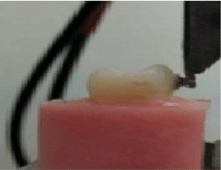
Figure 1: Shear bond strength measurement.
Adhesive remnant index: Adhesive remnant index was measured using image analyzer (JVC international Yokohama, Japan). Once the brackets were debonded, the enamel surface of each tooth was examined at 10X magnification in a stereomicroscope to determine the amounts of residual adhesive remaining on each tooth. The specimen was seated on a table under microscope and the (10x) lens selected. After scanning the specimen, image was captured and saved in an attached computer for analysis; areas with remnant adhesive were drawn using image analyzer software and measured (Figure 2). A modified adhesive remnant index (ARI) was used to quantify the amount of remaining adhesive, using the following scale: 1=all the adhesive remained on the tooth, with the imprints of the bracket base; 2=more than 90% of the adhesive remained on the tooth; 3=10% to 90% of the adhesive remained on the tooth; 4=less than 10% of the adhesive remained on the tooth, and 5=no adhesive remained on the tooth [2,28].

Figure 2: Method of measuring the adhesive remnant area using Image
analyser.
Tooth surface finishing using tungsten carbide bur and sandblasting: After measuring the ARI, The specimens of each group were divided into two groups, 15 each and adhesive remnant removal was performed using two different methods finishing burs Tungsten Carbide Bur (TCB) and Sandblasting (SB). The cleaned enamel surfaces were subjected to a test with a profile meter (kosaka Laboratory Ltd. Japan) to measure the enamel roughness. The profile meter has a tip that placed on the enamel surface touching the centre of each crown for all the measurements to scan and measure the surface roughness (Figure 3), two recordings made for each specimen and the mean values were taken. Three surface roughness measurements were recorded, the average roughness (Ra) indicates the overall roughness of the enamel surface. It is the arithmetic mean of all absolute distances of the surface roughness from the centre line within the measuring length. Root mean square roughness (Rq) describes the high distribution relative to the mean line and the maximum roughness depth (Rt) reflects isolated features on the enamel surface. The calibration of instrument was done following instructions and operation manual before each measurement.
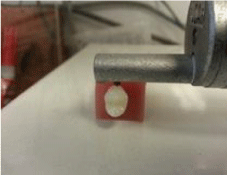
Figure 3: Enamel surface roughness measurement procedure.
Statistical analysis
Independent t test was used to determine whether there is a statistically significant difference in SPSS between the 2 test groups and to compare the bond failure mode (ARI scores) between the groups. Kurskal-Wallis test was used for Ra, Rq and Rt values analysis. Significance for all statistical tests was predetermined at P seis than 0.05.
Results
Shear bond strength (SBS) testing
The mean (SD) values of brackets debonding force using the universal testing machine for the 2 different enamel surface treatments, Sodium hypochlorite 5.25% + Conventional acid (Phosphoric acid 37%) and treatments with conventional acid (Phosphoric acid 37%) only were, 17 (± 5.37) and 13.86 (± 4.41) MPa respectively (Table 1). Statistically there was significant difference in the Shear Bond Strength value between the 2 groups P = 0.029.
95% confidence interval
Groups
n
Mean (SD) (MPa)
Lower Bound
Upper Bound
P value
Group I
30
17 (±5.37)
0.029
Group II
30
13.86 (±4.41)
-6.05
-0.227
n: Sample Size; Mean; SD: Standard Deviation
Table 1: Comparing SBS between group1 and II (independent t test).
Adhesive remnant index test
The mean (SD) score of adhesive (RMGIC) remained on the tooth surface of the 2 different enamel surface treatment groups (Sodium hypochlorite 5.25% + Conventional acid Phosphoric acid 37% and Phosphoric acid 37%, were 3.97 (± .718) and 2.90 (± .712) respectively (Table 2). Statistically there was significant difference between the 2 groups P > 0.001(Table 3).
Modified ARI score
Groups
n
1
2
3
4
5
Fuji Ortho LC with NaOCl
30
0
8
18
3
1
Fuji Ortho LC without NaOCl
30
0
0
8
15
7
n: Sample Size
Table 2: Frequency distributions of the modified ARI scores of the two groups.
Confidence Interval
Groups
n
Mean (SD)%
Lower Bound
Upper Bound
p- Value
Group I
30
3.97 (±.718)
-1.436
-0.697
>0.001
Group II
30
2.90 (±.712)
n: Sample Size
Table 3: Descriptive statistics for adhesive remnant index of the study groups.
Enamel surface roughness
The descriptive statistics for the surface roughness data of all specimens after adhesive removal using TCB and SB methods are shown in table 4. The median (IQR) of Ra (arithmetic average value of the profile departure from the mean line in the sampling length), Rq (Root of mean value, within measurement length) and Rt (Maximum Roughness) were:
Groups
Ra µm
Rq µm
Rt µm
Median(IQR)
Min
Max
Median(IQR)
Min
Max
Median(IQR)
Min
Max
Group I
TCB
.70(.11)
0.6
1.1
91(.19)
0.71
1.5
4.85(1.92)
0.6
9
SB
.75(.28)
0.5
1.5
1.00(.28)
0.6
1.6
5.65(2.65)
2.6
13.3
Group II
TCB
.65(.53)
0.4
1.5
.85(.55)
0.6
1.6
4.41(2.85)
2.6
8.7
SB
.70(.58)
0.5
1.5
.90(.65)
0.6
1.6
5.00(3.20)
2.6
8.7
TCB: Tungsten Carbide Bur; SB: Sandblast
Table 4: Results of surface roughness (μm) after finishing with TCB and SB methods.
Group 1: TCB were 0.70(.11), 0.91(0.19), 4.85(1.92) and SB 0.75(.28), 1.00(.28), 5.65(2.65) respectively and group 2: TCB were 0.65(0.53), 0.85(0.55), 4.41(2.85) and SB 0.70(0.58), 0.90(0.65), 5.00(3.20) respectively. Non parametric Kurskal-Wallis test was used for Ra, Rq and Rt values analysis. The results indicated that there are no significant differences among the 2 groups P value = 0.340, 0.483 and 0.280 respectively.
Discussion
Shear bond strength testing
The immediate bond strength of RMGIC, the mostly used adhesive material is far less than clinically acceptable limits [29,30], searching for a better material or modifying the procedure of bracket fixation is of great demand. Currently studies are focused on a faster bonding with harmless removal procedures and antibacterial effects of the bonding materials to aid in oral hygiene [31]. The present study have evaluated 2 enamel surface treatment using RMGI, Fuji Ortho LC to bond orthodontic brackets. The main objective of the study was to determine whether NaOCl, applied for 1 minute before etching, increase bracket SBS. By conditioning the enamel surface with 5.25% NaOCl followed by a 30-second etching with 37% phosphoric acid, the present findings indicated that the mean (SD) SBS of brackets bonded using Fuji Ortho LC is 17 (± 5.37) MPa, exceeds the clinical recommendation by [32], which is a minimum tensile bond strength of 5.9-7.8 MPa, in contrast, the Fuji group in which NaOCl was not used had a much lower mean SBS 13.86 (± 4.41). Our present study showed a higher mean SBS for both groups than most of other studies in which Fuji LC were used [33,34,35]. Luciana Borges, et al. [33] determined the influence of the light curing units on the shear bond strength of orthodontic brackets; they found that the mean SBS of the brackets bonded with Fuji Ortho with LED polymerization and 37% phosphoric acid etching is 5.49MPa, the groups were tested for shear strength in a universal testing machine at a crosshead speed of 0.5 mm/min. Justus, et al. [34] found the mean SBS for the brackets bonded using Fuji Ortho LC, with 37% phosphoric acid and enamel deproteinization is 9.64 ± 5.01 MPa, and the mean SBS for the brackets bonded using Fuji Ortho LC in the control group (without enamel deproteinization) was 5.71 ± 3.87 MPa, the results were statistically different at a crosshead speed of 1.0 mm/min. Tatiana Bahia et al [35] used 10% per cent polyacrylic acid as an etching material and Fuji Ortho LC as adhesive, the results showed that the mean (SD) SBS for the brackets bonded using enamel deproteinization is higher than that without the use of NaOCl, 9.86 ± 2.90 and 8.60 ± 5.29 respectively, however there was no statistically significant difference between the 2 groups at a crosshead speed of 0.5 mm/minute. It is very difficult task to compare between the studies, in this respect it is important to do standardization as possible as we can to avoid bias. There are many variables affecting the results of this study, such as adhesive, acid etch materials, polymerization of the material, time as well as different crosshead speed of Instron machines. We try to compare our study with relatively similar studies but unfortunately we found few studies exactly similar to ours.
From the above selected studies we found that there is a high difference in our mean SBS for control group (13.86) and the 3 three studies (5.49, 5.71 and 8.60), inspite of using the same adhesive system. This can be explained as there may be a missing factor that leads to this variation. Thus, if the clinician wishes to use RMGI to prevent white spot lesions, it is recommended, based on the findings of the present study, to deproteinize the enamel surface with 5.25% NaOCL for 1 minute before acid-etching as there was a statistically significant difference between the 2 Fuji Ortho LC groups. The control group showed an acceptable SBS, this can be explained by the fact that acid etching produces a well-defined etching pattern by preferential dissolution of either periphery or core of the enamel. The bonding agent used with Fuji LC has a very low viscosity and very high surface energy, which helps it to easily penetrate the fine etching pattern (2-5 µm in diameter) produced by acid etching and the application of NaOCL have an additional even lower etching ability, it increases the surface area by dissolving organic content of the enamel and give a chance to the etching material to penetrate more deeply in the core part of the enamel creating type 2 etching pattern. This explanation is in accordance with Justus, et al. [34], the authors considered that the application of NaOCl to achieve a better etching pattern is important, as well they have used scanning electron microscope (SEM) to study the enamel surface conditioned with NaOCl, they found that it produces a qualitatively rougher enamel surface and shows better etch pattern (types 1 and 2) than the images of the control group, in which NaOCl was not used (type 3 etch pattern), they concluded that, enamel deproteinization is an important step in the overall bracket bonding procedure, improve marginal seal of the bracket base to the enamel is obtained because of types 1 and 2 acid-etching patterns produced with the aid of the NaOCl application. White spot lesions (WSL) formation might be minimized due to this improved seal. They have referred the partial loss of organic elements on the enamel surfaces to the storage of the test specimens in distilled water. Thus, the authors believe that the in vivo application of NaOCl might result in greater SBS than demonstrated in this ex vivo study.
Adhesive ruminant index
Adhesive that was remain after debonding in the current study was higher than other studies who have used the same adhesive material (Fuji LC), we used gel type and not liquid and powder this matter is explained well by Sharma, et al. [1] as liquid to powder ratio of RMGIC is 3:1, which results in a very thick mix, difficult for this thick adhesive to penetrate the fine etching pattern (2-5 µm in diameter) produced by acid etching. Moreover we use LC which increases bond strength as mentioned by the LED curing unit also results in higher bond strength as compared to conventional halogen curing light [36]. Larmour and Stirrup, with Hegarty and (Hegarty and Macfarlane) [37,38], suggested that in the clinical situation, the use of Fuji Ortho LC may result in unacceptable bond failure rates, it should be noted that observation periods, materials (brackets, adhesives) and enamel surface conditioning widely differ from one study to another. In general, bond failure of brackets bonded using Fuji Ortho LC without NaOCl occurred at the enamel-adhesive interface, whereas brackets bonded using NaOCl failed more often at the bracket-adhesive interface, these results were significant [34]. Bracket failure at each of the 2 interfaces has its own advantages and disadvantages [38], brackets failure at the bracket-adhesive interface is advantageous as it indicates good adhesion to the enamel. However, considerable chair time is needed to remove the residual adhesive, with the added possibility of damaging the enamel surface during the cleaning process. In contrast, when brackets fail at the enamel-adhesive interface, less residual adhesive remains on the enamel but then bracket failure probably occurs more often during treatment, disrupting chair time and prolonging the duration of orthodontic treatment [34]. These results are similar to those reported by Espinosa, et al. [13]; etching of enamel with 37% phosphoric acid after eliminating the organic elements from the enamel surface probably produces longer adhesive tags that penetrate the enamel. The longer tags greatly increase the mechanical retention of adhesives to the enamel, particularly of RMGIs, as demonstrated in the present study. This adhesive requires a longer time to set than composite resin, and it has a lower SBS in the first half hour after bonding, although it increases 20-fold within the first 24 hours [27]. Thus, clinicians need to consider the properties of RMGIs to be able to use them successfully. It is hoped that manufacturers in the future will develop RMGIs with better initial bond strength. Because of the recent improvements in the fluoride-releasing capabilities and the SBS of RMGIs, it has been suggested that these adhesives should play a greater role (i.e., be more widely used) in bonding orthodontic brackets in the future [39].
Enamel surface roughness
Damage to the enamel can be attributed to the cleaning with abrasives before etching, acid etching, enamel fractures caused by forcibly removing brackets, or mechanical removal of remaining composite with rotary instruments [40]. In this study both methods TCB and SB qualitatively appeared to provide an acceptable and similar surface finishing following a pumice and brush polishing procedure after Fuji removal, and no statistically significant difference was observed between the two methods. This finding is in agreement with Banerjee and Watson [41], they have removed resin using these two methods. In Malaysia, Hasan, et al. [42] have found that the median roughness of intact enamel is 0 µm, and the best method among TCB, diamond, and green stone burs was cleanup with TCB after bracket debonding of 1 µm. In the current study, the median roughness of the enamel after debonding was lower than 0.65 µm using TCB. Cook, et al. [43] found that alumina airabrasion is effective in the removal of composite at a higher rate than sound enamel, indicating that this technique may be used to remove residual orthodontic adhesive resin on sound teeth. They stated that the success of air-abrasion in resin removal after bracket debonding is controlled by the use of an appropriate abrasive powder, the inherent characteristics of the air-abrasive stream, and the correct clinical technique applied. An ideal powder cannot cut into sound enamel but can successfully remove the residual resin adhesive. The inherent characteristics of air-abrasion, where by the divergent stream cuts at a higher rate at the centre than at the periphery, results from the laminar flow of the propellant gas. The characteristics provide the particles at the centre of the nozzle lumen a higher kinetic energy than the particles at the margins. This kinetic energy creates a less distinct abrasion margin, making the final surface easier to polish. To use these characteristics, the correct clinical technique has to be applied, which involves keeping the nozzle at a distance of at least 5 mm from the tooth surface. This distance allows the abrasive particle stream to diverge and create a less distinct abrasion margin. However, Banerjee and Watson [41], (2002) concluded that removal of adhesive using air abrasion caused more damage than that caused by the TCB, which was the gold standard used in their study. Moreover, the amount of enamel removed during adhesive removal using air abrasion was far less predictable than that removed by the TCB. This finding makes air abrasion an inappropriate clinical instrument for the removal of residual resin adhesive because of the inherent lack of substrate selectivity of air abrasion powder.
Conclusions
- Significantly greater bracket SBS can be obtained with Fuji Ortho LC if the enamel surface is wetted for 1 minute with 5.25% NaOCl, before etching.
- Applying 5.25% NaOCl to the enamel surface eliminates the organic elements. This effect allows the acid etchant to penetrate more effectively into the enamel, creating type I and 2 etching patterns.
- When enamel was deproteinized, larger amount of cement remained on the enamel surface in the group treated with acid etch.
- Surface enamel roughness after cleanup with TCB or SB represented by Ra, Rq and Rt are similar.
- Additional research is needed to determine the real clinical benefits of Na hypochlorite. In vivo testing of the effectiveness of RMGIs to prevent WSLs would be a worthwhile endeavor.
- Sharma P, Valiathan A, Arora A, Agarwal S. A comparative evaluation of the retention of metallic brackets bonded with resin-modified glass ionomer cement under different enamel preparations: A pilot study. Contemp Clin Dent. 2013; 4: 140-146.
- Bishara SE, VonWald L, Olsen ME, Laffoon JF. Effect of time on the shear bond strength of glass ionomer and composite orthodontic adhesives. Am J Orthod Dentofacial Orthop. 1999; 116: 616-620.
- Choo SC, Ireland A, Sherriff M. An in vivo investigation into the use of resinmodified glass poly (alkenoate) cements as orthodontic bonding agents. Eur J Orthod. 2001; 23: 403-409.
- Wheeler AW, Foley TF, Mamandras A. Comparison of fluoride release protocols for in-vitro testing of 3 orthodontic adhesives. Am J Orthod Dentofacial Orthop. 2002; 121: 301-309.
- Canay S, Kocadereli I, Akça E. The effect of enamel air abrasion on the retention of bonded metallic orthodontic brackets. Am J Orthod Dentofacial Orthop. 2000; 117: 15-19.
- Buonocore, M. G. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J Dent Res.1955; 34: 849-853.
- Carstensen, W. Effect of reduction of phosphoric acid concentration on the shear bond strength of brackets. Am J Orthod Dentofacial Orthop.1995; 108: 274-277.
- Kinch AP, Taylor H, Warltler R, Oliver RG, Newcombe RG. A clinical trial comparing the failure rates of directly bonded brackets using etch times of 15 or 60 seconds. Am J Orthod Dentofacial Orthop.1988; 4: 476-483.
- Graber TM, Eliades T, Athanasiou A. Risk management in orthodontics: experts’ guide to malpractice. Br Dent J. 2004; 198: 114-115.
- Fjeld, M, øgaard B. Scanning electron microscopic evaluation of enamel surfaces exposed to 3 orthodontic bonding systems. Am J Orthod Dento facial Ortho. 2006; 130: 575-581.
- Ercan E, Özekinci T, Atakul F, Gül K. Antibacterial activity of 2% chlorhexidine gluconate and 5.25% sodium hypochlorite in infected root canal: in vivo study. J ENDOD. 2004; 30: 84-87.
- Grandini S, Balleri P, Ferrari M. Evaluation of Glyde File Prep in combination with sodium hypochlorite as a root canal irrigant. J Endod. 2002; 28: 300-303.
- Espinosa R, Valencia R, Uribe M, Ceja I, Saadia M. Enamel deproteinization and its effect on acid etching: An in vitro study. J Clin Pediatr Den. 2008; 33: 13-19.
- Penido SM, Penido CV, dos Santos-Pinto A, Gandini LG Jr, Bagnato VS. In vivo and in vitro study of the shear bond strength of brackets bonded to enamel using halogen or LED light. World J Orthod. 2009; 10: 21-28.
- Yamamoto A, Yoshida T, Tsubota K, Takamizawa T, Kurokawa H, Miyazaki M. Orthodontic bracket bonding: enamel bond strength vs time. Am J Orthod Dentofacial Orthop. 2006; 11130: 435.
- Zachrisson BU, Buyukyilmaz T. Bonding In Orthodontics. In Graber TM, Vanarsdall R L, Vig KWL. (Eds.) Orthodontics: Current Principles and Techniques. 4th ed. Elsevier Mosby. 2005; 579 - 660.
- Pus MD, Way DC. Enamel loss due to orthodontic bonding with filled and unfilled resins using various clean-up techniques. Am J orthod. 1980; 77: 269-283. Powers JM, Kim HB, Turner DS. Orthodontic adhesive and bond strength testing. Semin Orthod. 1997; 3: Elsevier pp. 147-156.
- Rouleau BD Jr, Marshall GW Jr, Cooley RO. Enamel surface evaluations after clinical treatment and removal of orthodontic brackets. Am J orthod. 1982; 81: 423-426.
- Campbell PM. Enamel surfaces after orthodontic bracket debonding. Angle Orthod.1995; 65: 103-110.
- Hong Y, Lew KK. Quantitative and qualitative assessment of enamel surface following five composite removal methods after bracket debonding. Eur J Orthod.1995; 17:121-128.
- Howell S, Weekes WT. An electron microscopic evaluation of the enamel surface subsequent to various debonding procedures. Aust Dent J. 1990; 35: 245-252.
- Burapavong V, Marshall G, Apfel D, Perry H. Enamel surface characteristics on removal of bonded orthodontic brackets. Am J Orthod. 1978; 74: 176-187.
- Smith S, Walsh LJ, Taverne AA. Removal of orthodontic bonding resin residues by CO2 laser radiation: surface effects. J Clin Laser Med Surg.1999; 17: 13-18.
- Thomas BW, Hook CR, Draughn RA. Laser-aided degradation of composite resin. Angle Orthod.1996; 66: 281-286.
- Hannemann M, Diedrich P. Der Einsatz des Prophy-Jet®-Gerätes zur Schmelzpolitur nach der Bracketentfernung. Fortschr Kiefer orthop.1986; 47: 317-326.
- Wright GZ, Hatibovic-Kofman S, Millenaar DW, Braverman I. The safety and efficacy of treatment with air abrasion technology. Int J Paediatr Dent. 1999; 9: 133-140.
- Diedrich P. Enamel alterations from bracket bonding and debonding: a study with the scanning electron microscope. Am J orthod. 1981; 79: 500-522.
- Bishara SE, Ostby AW, Laffoon J, Warren J. Shear bond strength comparison of two adhesive systems following thermocycling. Angle Orthod. 2007; 77: 337-341.
- Boersma JG, van der Veen MH, Lagerweij MD, Bokhout B, Prahl-Andersen B. Caries prevalence measured with quantitative light-induced fluorescence after treatment with fixed orthodontic appliances: influencing factors. Caries Res. 2005; 39: 41-47.
- Stecksén-Blicks C, Renfors G, Oscarson ND, Bergstrand F, Twetman S. Caries-preventive effectiveness of a fluoride varnish: A randomized controlled trial in adolescents with fixed orthodontic appliances. Caries Res. 2007; 41: 455-59.
- Algera TJ, Kleverlaan CJ, Prahl-Andersen B, Feilzer AJ. The influence of different bracket base surfaces on tensile and shear bond strength. Eur J Orthod. 2008; 30: 490-494.
- Reynolds IR. A review of direct orthodontic bonding. Br J Orthod 1979; 2: 171-178.
- Retamoso LB, Onofre NM, Hann L, Marchioro EM. Effect of light-curing units in shear bond strength of metallic brackets: an in vitro study. Appl Oral Sci. 2010; 18: 68-74.
- Justus R, Cubero T, Ondarza R, Morales F. A New Technique with Sodium Hypochlorite to Increase Bracket Shear Bond Strength of Fluoride-releasing Resin-modified Glass Ionomer Cements: Comparing Shear Bond Strength of Two Adhesive Systems with Enamel Surface Deproteinization before Etching. Semin Orthod. 2010; 16: 66-75.
- Tatiana Bahia Junqueira Pereira, Wellington Corrêa Jansen Matheus Melo Pithon, Bernardo Quiroga Souki, Orlando Motohiro Tanaka and Dauro Douglas Oliveira. Effects of enamel deproteinization on bracket bonding with conventional and resin-modified glass ionomer cements. The European Journal of Orthodontics Advance Access published February. 2012; 2: 1-5.
- Wendl B, Droschl H. A comparative in vitro study of the strength of directly bonded brackets using different curing techniques. Eur J Orthod. 2004; 26: 535-544.
- Larmour CJ, Stirrups DR. An ex vivo assessment of a resin-modified glassionomer cement in relation to bonding technique. J Orthod. 2001; 28: 207- 210.
- Hegarty DJ, Macfarlane TV. In vivo bracket retention comparison of resinmodified glass ionomer cement and a resin-based bracket adhesive system after a year. Am J Orthod Dentofacial Orthop. 2002; 121: 496-501.
- Eliades T. Orthodontic materials research and applications: Part 1. Current status and projected future developments in bonding and adhesives. Am J Orthod Dentofacial Orthop. 2006; 130: 445- 451.
- Hosein I, Sherrif M, Ireland AJ. Enamel loss during bonding, debonding, and cleanup with use of a self-etching primer. Am J Orthod Dento facial Orthop. 2004; 126: 717-724.
- Banerjee A, Watson TF. Air abrasion: its uses and abuses. Dent Update. 2002; 29: 340-346.
- Hassan D, Rozita H, Normastora A. Study of shear bond strength between trans bond XT with SEP and fuji ortho LC and enamel surface roughness after resin removal with three different finishing burs. 2010; 68-72.
- Cook R, Azzopardi A, Thompson I, Watson TF. Real-time confocal imaging, during active air abrasion–substrate cutting. J Microsc. 2001; 203: 199-207.
The increased bonding strength allows the orthodontist to use fluoride-releasing RMGIs as bonding adhesives to be able to possibly protect enamel from developing WSLs, which is a major iatrogenic effect of orthodontic treatment. Combining clinical aims and experience with the best available evidence should be an important goal of every clinician.
References