
Research Article
J Dent & Oral Disord. 2023; 9(1): 1178.
The Efficacy of Fluoride Rinse on Caries Increment, Plaque Occurrence and Gingival Status in Children Undergoing Orthodontic Treatment. A Randomized Controlled Clinical Trial with Results after 6- and 12 Months
Ekstrand KR1*; Tronier-Knowlton J2; Mikkjalsdóttir R2; Fedders SB2; Heidke R2; Sonnesen L2
1Section of Cariology, and Endodontics Department of Odontology, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark
2Postgraduate Education in Orthodontics Department of Odontology, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark
*Corresponding author: Kim Rud Ekstrand Section of Cariology, and Endodontics, Department of Odontology, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark, Nørre Alle 20, 2200 Copenhagen N. Denmark. Tel: +4524431177 Email: kek@sund.ku.dk
Received: September 16, 2023 Accepted: October 11, 2023 Published: October 18, 2023
Abstract
This triple blinded RCT two arms study determines the efficacy of fluoride rinse (0.32% NaF-solution) for a period of 6 and 12 months (6m,12m) on the caries increment, plaque occurrence and gingival conditions in 10-15 year old Danes undergoing orthodontic treatment. Estimations showed that 24 subjects were required in the Test Group (TG) as well as in the Control Group (CG) at the end of the study. A total of 61 subjects were randomly assigned to TG (n=30) and the CG(n=31). Participants rinsed twice a week with 10 ml NaF solution (TG) or 10 ml of a placebo solution (CG). One assessor recorded at baseline, after 6-and 12 months caries (ICDAS index) and plaque and gingival status (Løe index). Four participants in each group dropped out during the study period. At patient level 27% in TG and 59% in CG showed caries progression during 12 month (p<0.05). Parametric tests confirmed that CG-participants had significant higher progression rate than TG-participants from 0m to 12m (p=0.014). The preventive fraction was 77% in favour of the TG-participants. Similar calculations from baseline to 6 months after, there were no significant caries increment difference between TG and CG. At 0m and 12m there was no significant difference in the plaque index and the gingival index between the two groups (p-values>0.05). The study conclusion was that rinsing twice a week with a 0.32% NaF solution during orthodontic treatment with fixed appliance had a significant effect on caries progression in children and adolescents during a treatment period of 12 months.
Keywords: Caries; Orthodontic treatment; Prevention; White spot lesions; Fluoride rinsing
Introduction
Denmark is known for that children and young people has a very low caries experience [1, 2]). This is, among other things, due to that in Denmark there is a focus on brushing teeth with fluoride-containing toothpaste, from the emergence of the 1st primary tooth and that Denmark has for the last 50 years had a Public Dental Health Care System free of charge covering the group of 0 to 18 years of age. The scheme also applies to orthodontic treatment for those approximately 25% of the child and youth population, who has the most severe functional disorders in their dentition. The literature shows that a combination of using fluoride toothpaste every day and fluoride rinses every day (low concentration, ≤250 ppm F-) or a few times a week (high concentration, ≥900 ppm F-) has a greater preventive effect than using fluoride toothpaste alone, in patients with moderate to high caries risk [3].
It is well known that orthodontic treatment increases the risk of developing caries, especially the cosmetically disfiguring facial lesions that develop due to the artificial plaque stagnation areas around bands and brackets [4-6]. These lesions can be difficult to mask after the orthodontic treatment [5,6].
Data from a review study [7] indicated that cementation materials containing fluoride, and low fluoride containing solutions for rinsing have a caries reducing effect on patients, who were under orthodontic treatment. However, bias was a prominent feature in the studies that have been conducted up to 2005 [7]. A Dutch RCT study [5] testing the effect of a low fluoride containing solution compared to a placebo solution showed a significant effect in reducing the increment of white spot lesions on patients under orthodontic treatment. In a Swedish RCT study [8], where the testing product was a fluoride containing varnish versus a placebo varnish applied every 6 weeks, during the orthodontic treatment an 14% less caries increment was noted (p> 0.05) in the test group compared to the control group. When expressed at the severity level the difference became significant in favor for the fluoride containing product.
There is no information about the incidence of caries in children and young people in Denmark as a consequence of receiving orthodontic treatment and no information whether a high concentrated fluoride rinses during the orthodontic treatment, twice a week, could reduce the incidence of caries. Further, it is important to mentioned that it is not legal in Denmark to use fluoridated toothpaste with > than 1500 ppm fluoride below the age of 16.
This study investigates whether fluoride rinses, twice a week, with a solution containing 0.32% Sodium Fluoride (NaF) ~1450 ppm fluoride effects on the caries increment, the plaque accumulation and gingival status in patients during the first year of orthodontic treatment with fixed appliances.
Thus, the primary outcome was to test any difference between test- and control group in increment of caries, at patient level and on group level, related to teeth with fixed appliances from baseline (0m) during 6 months (6m)- and 12 months (12m).
The two secondary outcomes were to test any difference between test- and control group in plaque occurrence and gingival status on 12 index teeth at baseline (0m) during 6m and 12m with orthodontic treatment.
Benson et al. stated in 2005 [7] that new studies in the present field of research should follow a protocol focusing on controlling for bias. There are several types of bias where the majority can be controlled for constituting a problem, if the study is planned well. We have taken the liberty to discuss the different types of bias used in Cochrane reviews of RCT-studies [see for example 3] related to the present study.
Material and Methods
The project has been approved by the Danish Ethics Committee (H-19062827) and by the Data Protection Authority (514-0446/19-3000).
Duration
The first participant got the baseline clinical examination December 20. 2019 and all participants had their baseline clinical examination finalized October 26. 2020. The data for the last 12 month clinical examination was November 4. 2021.
Location
The study took place at the School of Dentistry in Copenhagen at the Department of Orthodontics.
Trial Design
Triple blind randomized clinical trial with two parallel arms.
Products
The test products contain a 0.32% NaF-solution in bottles of 500 ml. The placebo was a liquid consisting only of water, but also in 500 ml bottles.
Inclusion Criteria
Medically healthy/or with minor disabilities aged 10-15 years, who need orthodontic treatment with fixed appliances.
Exclusion Criteria
Patients with chronic diseases who receive poly-pharmacy.
Sample Size
Based on β of 80% and a of 5% and an average difference of 2.5 lesions with a spread of 3 lesions, the Quick form shows 16/(2.5/3)² = 24 patients in each group [9]. As it was estimated that 5 patients will drop out during the study period, the aim was to allocate 30 patients in each group.
Randomization
The sample frame was 10-15 year olds in great Copenhagen area who were offered orthodontic treatment with fixed appliances in the local Child Dental Health surface and referred to the orthodontic department of the Dental School in Copenhagen, University of Copenhagen. A total of 68 candidates were addressed and from these 61 children/adolescents were interested in participating in the study, and both parents signed the consent form (Table 1).
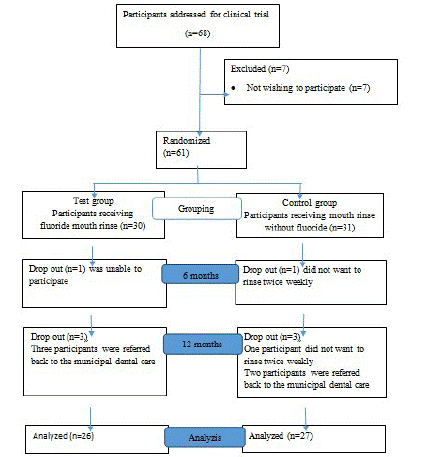
Table 1: Flowchart, showing participants addressed for clinical trial, randomization, grouping, attrition and the number of participants in the test- and control group after 12 months.
The allocation to the test- or the control group was made by draw a piece of paper with a preprinted number between 1 and 61. This procedure was orchestrated by two of the co-authors Fedders SB, Heidke R, and they pass the results to the secretary at the Department. She made sure, based on her list, who among the participants got either the fluoride containing product (test group) or the placebo (control group).
Program
The orthodontic treatment of the participants was handled by 4 trainee dentists (all co-authors to this study) seeking specialization within orthodontics all under the supervision of an experienced specialist dentists (Last author).
Initial, Fedders and Heidke made sure that two Bite-wings were taken and stored at the electronic file system. The participants and their parents were instructed and re-instructed in a toothbrushing technique related to which appliance the participant was treated with. In addition, the participant and the parents were instructed that the participant should rinse the mouth with 10 ml of the liquid in the supplied bottle twice a week for 45 seconds, after which the liquid should be spit out. Further, the participants should avoid eating and drinking for the next half hour after rinsing.
Recordings
All recordings relevant for the study were made by one assessor (the first author). The assessor was blind to what group the participants were allocated to during the whole study.
The condition of plaque and gingiva was recorded using Løe's scoring systems [10]
Caries was recorded using the ICDAS, which uses 7 clinical scores, including activity determination [11]. The bite-wings were also scored using the D1-4MFS index. (radiolucency in 1 = outer enamel, 2 = inner enamel and outer 1/3 of the dentine, 3 = middle 1/3 of the dentine and 4 =inner 1/3 of the dentine [12].
Clinical Examination
The assessor performed the clinical examination on the participants placed in a dental chair. The presence of plaque and gingival status was recorded on 12 index teeth (first permanent molar teeth and maxillary and mandibular incisors). It was recorded on the facial surface of the teeth in question.
The following registration was used clinically for plaque occurrence [10]:
0: No plaque
1: A thin layer of plaque along the gingival margin is detected by probing
2: Moderate layer of visible plaque along the gingival margin. Proximal spaces are free of plaque but plaque is visible to the naked eye
3: Greater amount of plaque along the gingival margin and in the proximal space
The following gingival index is used clinically for gingival status [10]:
0: Healthy gingiva
1: Gingival redness without bleeding on probing
2: Gingival redness and bleeding on probing
3: Ulceration and spontaneous bleeding
The following method and criteria was used clinically for recording caries:
After the plaque and gingival registrations, the assessor brushed the participant's teeth with a traditional toothbrush or with a solo toothbrush (without toothpaste). Next, cotton roles were placed to control for saliva secretion from the parotic glandular and teeth were dried with the three-in one syringe. All teeth that were to have fixed appliances were registered. Evidence of caries was recorded on the facial surface of the tooth. The examination took place under optimal clinic lighting, and a probe was used to gently identify breaks, and texture in terms of the enamel was rough or smoot to probing.
The ICDAS Classification system (Ekstrand et al 2007) [11]
1. Healthy tooth surface. No evidence of caries after 5 seconds of air drying
2. First visual change in enamel: Opacity or discoloration (whitish 1W or brownish, 1B) is visible after pronounced air drying
3. Cariological changes in the enamel without drying out (White 2W or brownish 2B)
4. Localized breakdown of enamel, no visible dentin
5. Underlying dark shadow from carious dentin, with or without localized breakdown of the enamel
6. Cavity with visible dentin, cavity involving less than half of the dental surface
7. Pronounced cavity with visible dentin, cavity deep and wide involving more than half of the tooth
The ICDAS system also investigated whether the observed lesion was active or arrested (Ekstrand et al. 2007) [11]. To determine this, it was recorded whether or not the gingiva close to the lesion was inflamed (Ekstrand et al., 1998) [13] and whether the lesion felt rough or smooth on probing. In the case of a cavity, it was assessed whether the dentin was soft or hard by probing.
Definition of active and arrested lesions in this study by modum Ekstrand et al. (2007) [11].
Active lesion:
• Whitish, rough on probing with or without bleeding from the gingiva
• Whitish, smooth on probing, bleeding from gingiva
• Brownish, rough on probing, bleeding from gingiva
• Arrested lesion:
• Brownish, rough on probing, healthy gingiva
• Brownish, smooth on probing with bleeding from the gingiva
• Whitish, smooth on probing, healthy gingival
Intra-Reliability Recordings
The assessor achieved in another clinical study beginning in 2019 [14] an intra–examiner kappa value on 0.92 (95% CI0.89, 0.94) using the ICDAS index on examining11 children. Bite-wing radiographs were also analyses on the 11 children and the assessor, at the present study, achieved an intra-examiner kappa value on 0.84 (95% CI0.77, 0.91 on using the same radiographically scoring system used also in the present study.
Question
The participants were asked twice by the assessor during the study (6m and 12m) if they had any complaints to the solution they rinsed with.
Data Handling
During the study all clinical scores (teeth undergoing orthodontic treatment, plaque-, gingiva- and caries scores were written down on paper copies on a form used in the Child Dental Health service in Denmark for recording the dental status (Figure 1) [15]. A form was created in the excel sheet, where the students marked which teeth were covered by fixed appliances. For this 12 month study the first author transferred all data obtained from the paper form, every 3 months until the end of the clinical assessments to the excel sheet. During this work, the first author was blinded to which group the participants were allocated to. The final dataset involving data from baseline, after 6 months and to the 12 months with orthodontic treatment was forwarded the company mentioned below, on the prepared excel files by the first author.
Statistical Considerations
All statistical calculations were done by a private company (MATx). The person in the company who did the statistical calculations was blinded to which group the participants were allocated to. Descriptive statistics (numbers, means, SD, medians) to illustrate tendencies in the two groups concerning caries increments, plaque accumulations and gingival status, after 6m and after 12m during orthodontic treatment. Plots for normality of the datasets using Kolmogorov-Smirnov tests [16] showed that caries data and data about gingival status could be considered as not deviating from being normally distributed (p-values<0.05). Plaque data on the other hand were not normally distributed (p>0.05). Data which were normally distributed were treated by parametric statistics (t-test, related or unrelated), while Wilcoxon matched-pairs signed-rank test (related samples) or Mann-Whitney U test (for independent samples) were used for data set not normally distributed [16]. A Generalized Estimation Equation model was created to investigate if one or more of the following 5 explanatory variables influence on the 12 months caries outcome data: GROUP (1 or 2), GENDER (1 or 2), AGE (between 10 and 15), EXTEND OF FIXED APPLIANCES (1=involved less than 10 teeth, 2=involved from 10 to 19 teeth, and 3=>19 teeth and CARIS RISK in permanent teeth based on reading the baseline radiographs (1=low risk (no caries lesions/restorations) or 2= high risk (any lesions/restorations). The probability distribution was the Poisson [9]. Finally, the preventive fraction using the caries increment in the 2 groups from baseline to 12 months with 95% confidence interval was calculated. Statistical analyses were performed by using IBM SPSS 20.0. A significant level <5% was considered as being significant.
Results
Descriptive Statistics
Initially, 68 participants were approached (Table 1), where 61, both parents signed the consent form. Of the 61 participants 30 were randomly allocated to the test- and 31 to the control group. One participant was lost within 6m in both groups, and further 3 were lost after 6m but before 12m in both groups leaving 26 and 27 participants in the test- and control group, respectively (Table 1).
At baseline the mean age of the participants was in the test group 13.5 years (1SD=1.29) and in the control group 13.3 years (1SD=1.14) (p=0.545).
At baseline about 1/3 of the participants were boys (33,9%), and about 2/3 were girls (66,1%). There was no gender difference at baseline in the two groups (p=0.858), neither during the study.
Analyzis of the bitewing radiographs showed that 1 participant in the test group had a D3 lesion requiring a restoration, while 2 others had 2 and 4 D1-2 lesions (enamel lesions) and 1 had an occlusal restoration. Similar figures in the control were that 2 participants had 1 to 3 D1-2 lesions and 2 had one occlusal restauration.
Primary Outcome
In both groups we can see that the absolute number of lesions increases during the study (Table 2, third column) but at a much higher increment rate in the control group (from 13/47/69) than in the test group (8/19/20).The statistical analyses within the groups (T-tests paired, last column, Table 1) disclosed that there was no significant difference in the increment rate from baseline (0m) to 6m, from 6m to 12m or from 0m to 12m in the test group. In contrast the increment rate increases significantly during the study in the control group (p-values<0.05, Table 2).
N
Number of lesions rela-ted to teeth, which should have or got fixed appliance.
Number of participants with lesions are also mentionedMin.
Max.
Mean
SD
T-tests related
P-valuesTest Baseline (0m)
30
8 lesions
in 3 participants0
2
0.28
0.92
0m-6m,
p=0.086Test 6 months (6m)
29
19 lesions
in 8 participants0
5
0.66
1.32
6m-12m, p=0.212
Test 12 months (12m)
26
20 lesions
in 9 participants0
7
0.77
1.58
0m-12m,
p= 0.117Control Baseline (0m)
31
13 lesions
in 6 participants0
3
0.43
0.97
0m-6m,
p=0.022Control 6 months (6m)
30
47 lesions
in 11 participants0
7
1.57
2.76
6m-12m, p<0.001
Control 12 months (12m)
27
69 lesions
in 17 participants0
13
2.56
3.22
0m-12m, p<0.001
Table 2: Data on number of lesions, lowest (min.) and highest number (max.) of lesions, mean number of lesions and the Standard Deviation (1SD) in the test- and control groups, divided into 0 months (0m), 6 months(6m) and 12 months(12m). P-values are presented.
Further statistical analyses between the groups (T-tests unrelated, Table 2 last row) showed that there was no difference at baseline (p=0.525), or after 6m (p=0.11), while after 12m wearing the fixed appliances, the caries increment rate in the control was significant higher than in the test group (p=0.014).
Analyses at the individual level disclosed that 19(73.1%) individuals were scored with less or the same caries status and n=7(26.9%) with higher caries status after 12m compared to 0m in the test group. Similar data in the control group was 11 individuals (40.7%) had less or the same caries status and n=16 (59.3%) had higher caries status after 12m compared to 0m (Chi-square, 5.638, Df=1, p=0.018).
The GEE model revealed that apart from the explanatory variable, GROUP (group 1 the fluoride rinsing group; group 2 placebo rinsing group, p=0.006) none of the others explanatory variables were associated to the caries outcome after 12m (p≥0.05).
The preventive fraction (caries increment in the test group, 0.77-0.28=0.49, caries increment in the control group 2.56-0.43=2.13) was 0.49-2.56/2.56=-0.77 (see data in Table 2) with a 95% confidence interval on -0.96 to -0.58.
Secondary Outcomes
Table 3 shows data about plaque indexes in the test- and control groups at 0m, after 6m- and 12m wearing the fixed appliances. In both groups the plaque index increases from 0m to 6- and 12m, apart from in the test-group after 6m, where the median (0.17) was surprisingly low. In the test-group the increase was not significant at any time, while between 0m and 6m, and between 0m and 12m significant values were obtained in the control group and borderline significance from 6m to 12m (p=0.054).
Test–versus control
Plaque
0m
6m
12m
p-values
WilcoxonTest
Mean
1SD
Median0.39
0.31
0.330.43
0.58
0.170.50
0.50
0.38t0m-t6m p= 0.684
t6m-t12m p=0.094
t0m-t12m p=0.457Control
Mean
1SD
Median0.43
0.43
0.330.56
0.46
0.430.72
0.52
0.72c0m-c6m p= 0.019
c6m-c12m p<0.054
c0m-c12m p<0.001Mann-Whitney
p-values
t0m- c0m
p=0.249t6m- c6m
p=0.157t12m-c12m
P=0.075
Table 3: Plaque data expressed as means, Standard Deviation (1SD) and the median in the test- and control groups, divided into 0m, 6m and 12m. p-values are presented.
Between the groups there were no significant values, but after 12m wearing the fixed appliances, the control group had an index closed to be significant higher than the index was in the test-group (p=0.075).
Table 4 shows data about the gingival status in the test- and control groups at 0m (baseline), after 6- and 12m wearing the fixed appliances. Looking at the means an increase in the gingival status is noted from 0m after 6- and 12m in the test-group, but only significantly from 6m to 12m. A significant increase in gingival index status was seen from 0m to 12m (means 0.37, 0.51, p=0.013) in the control group, while the indexes were the same between 0m and 6m (means0.36/0.37). Between the groups there were no significant values during study period.
Test- versus control
Gingival status
0m
6m
12m
p-values
T-tests relatedTest
Mean
SD0.16
0.20.18
0.340.29
0.37t0m-t6m p= 0.84
t6m-t12m p=0.005
t0m-t12m p=0.112Control
Mean
SD0.37
0.40.36
0.510.51
0.48c0m-c6m p= 0.161
c6m-c12m p=0.132
c0m-c12m p=0.013T-test unrelated
p-values
p=0.7
p=0.184
p=0.157
Table 4: Data concerning gingival status expressed as means and the Standard Deviation (SD) in the test- and control groups, divided into 0m, 6m and 12m. P-values are presented.
Side Effects to the Rinsing Solutions
When the participants were asked, at the clinical assessments after 6- and 12 months by the assessor, none of the participants reported any side effect of the rinsing solutions (whether the solution contained fluoride (test) or only water (control) apart from 1, who mentioned a metallic feeling, when the participants rinsed with the solution.
Discussion
The purpose of this study was to investigate whether fluoride rinsing (~1450 ppm F) twice a week, on low caries risk (see data from the radiographs) children and adolescents undergoing orthodontic treatment, had a caries-preventive effect.
The study design was a triple- blind randomized clinical trial.
It was planned that the study should finalize after each participant had ended the orthodontic treatment, but due to the Corona crises, we noticed in the summer period of 2021 that we lost participants because the referring Child Dental Health Services in the municipalities did start to call the participants home to finalize the orthodontic treatment in the home municipality. So instead of ending during 2022, with too few participants we decided to stop the recordings after 12 months where the participants had worn fixed appliances. This decision was told to the participants and their parents.
The primary outcome was related to the increment rate of caries lesions in the two groups, and the two secondary outcomes focused on plaque occurrence and gingival status in the two groups.
The three outcome parameters were recorded at baseline (0m), after 6 months (6m) and after 12 months 12m) wearing fixed appliances using the well described ICDAS index (11,12] and Løe’s plaque- and gingival indices [10].
In this study one very experienced assessor did all the clinical recordings, as well as reading the baseline bite-wing radiographs, but no registrations for calculating the assessors intra reproducibility were done on the sample in question. That was due to the fact that the assessor in a parallel RTC study to the present one, achieve an excellent intra examiner agreement in caries recording with the ICDAS both clinically and radiographically (Kappa- values > 0.8 [14].
In absolute numbers, 3 from the test group had 8 active lesions at baseline increasing to 19 in 8 participants after 6m to 20 in 9 (35%) participants after 12m with fixed appliance. In the control group, 6 participants had a total of 13 lesions at baseline, increasing to 47 in 11 participants after 6m which increased to 69 lesions involving 17 (63%) participants after 12m. No significant difference in caries increment was noted between the 2 groups after 6 months.
All lesions in the test group were registered as 1W, while in 2 participants in the control group the lesions had progressed to 2W lesions from the 6 months recording to 12 months recording. Neither in the test nor in the control group were active lesions with cavity development (ICDAS score =3) observed.
The study showed a significant effect of fluoride rinses on reducing the increment of white spot lesions (p=0.014), corresponding to a preventive effect of 77%. This means that the increment in the test group was 77% less in the test group compared to the control group from the start of the study to 12 months with fixed equipment in both groups. None of the explanatory variables apart from the GROUP (p=0.006) were significantly associated to the primary outcome.
Two prevalence studies [17,18] found that 38- and 46% and 40- and 43% of adolescents under orthodontic treatment had developed one or more lesions after 6 and 12 months, respectively. Similar data in our control group was 41 and 61%, not far from the above mentioned data. All studies illustrate that caries development on children and adolescents who achieve orthodontic treatment rather quickly, at a visible stage, develops lesions for example within less than 6 months. Lesions can also develop in the longer run, as seen in 1 and 6 participants in our study who developed lesions after 6 months in the test- and control group, respectively.
The study documents what Thylstrup et al., (1994) [4] stated back in time that caries to be developed requires a plaque stagnation area, which orthodontics appliances seems to create.
Our data are also largely consistent with data from the Dutch study [5] also investigating the effect of rinsing with a fluoride containing solution. They found that in their test group, 31% developed one or more white spot lesions during their study, against 47% in the control group. The corresponding data in the present study were 27% and 59%.
There were some differences between the 2 studies. The Dutch study [5] used a solution on 250 ppm fluoride and the rinsing had to be performed every day. In the present study we used 0.32% NaF solution, which is the highest concentration of fluoride, which is legal in Denmark, without a prescription. Due to the high concentration of fluoride we followed the advice from another studied [3] that the participants had to rinse only twice per week. In the Dutch study the increment of caries was investigated when the orthodontic treatment was finished, thus more than 2 years (mean 24.5 months) after the treatment had started. In the present study we examined the increment after wearing the orthodontic appliances in on 6 and 12 months.
Research indicates that fluoride in high concentrations has an effect on the composition of the plaque [19], which is why it could be expected that the plaque accumulation was less in the test group compared to the control group during the orthodontic treatment and that this would likely also affect the gingival condition in the two groups. Although there was a marked difference in plaque score between test- and control group after 12m (median 0.33 in the test group versus 0.72 in the control group, Table 3), the statistics analyzes showed that the level was not significant (p=0.075). The same relationship applied to the gingival conditions (mean in the test group 0.29 versus mean in the control group 0.59 p=0.157, Table 4).
It is important to remember that the sample size was calculated based on expected increment in caries and not on differences in plaque/gingival status as they were secondary outcomes. A larger sample could possibly have documented if fluoride rinses had a plaque-reducing effect, as table32 indicated a rather strong tendency, as described in the literature [19].
There is also some financial considerations to consider. As, at least in Denmark, there is no major difference in cost to prepare a solution of fluoride on 0,05%- or 0.32% NaF, 0,05% solution is more expensive as the participants has to rinse daily, compared to twice a week if they used 0.32% NaF solution.
Supplementary Information
Random Sequence Generation and Allocation Concealment (Selection Bias)
The authors had no influence on the sample frame, as it consisted of patients in need of orthodontic treatment, referred from the Child Dental Health Service in Great Copenhagen to the orthodontic department at the Dental School.
The participants, after the parents had signed the consent form (n=61), were allocated to either the test- or the control group by drawing a number between 1 and 61. The secretary had a pre-established list where numbers 1-61 each indicated that the participants should have a bottle with a green circle or no green small circle on. The secretary was blind to know what was in the bottles in terms of fluoride or placebo. Thus, we consider that the selection bias is under control.
Blinding of Participants and Personal (Performance Bias)
The participants, the dentists doing the orthodontic treatment, the assessor, the secretary and the external persons who did the statistical assessment were all blind to what group the participants were in to (triple blinded). Thus, we consider that the performance bias was under control.
Blinding of Outcome Data (Detection Bias)
The two trainee dentists Fedders and Heidke, opened the codes after 6 months to treat the data, from baseline to 6 months as that should be used as a part of the test to achieve the specialization degree. Similarly the two other trainee dentists Tronier-Knowlton and Mikkjalsdóttir followed the same procedures, but after12 months, also to achieve the specialization degree. The first author was not aware of whom were in the test- or in the control group before the codes were broken after the statistician had treated the data. Thus, we consider that the detection bias was under control, as the final data set forwarded to the statistician was done by the first author after the 12 months examinations.
Incomplete Outcome Data (Attrition bias)
A total of 8 participants, 4 from each group, were lost during the 12 month period, that this study covers. This leaving 2- and 3 participants more than the sample size required to be participating at the end of the study for the data should not be underpowered. One drop out in each group was scored based on the baseline bitewing as being in high risk. Similarly, data in both the test- and control group was 19%. Further, 1 of the 4 participants in the test group who dropped out during the study had an active lesion at baseline, versus 3 participants among those who stayed in the study in the test-group. Thus, there were no major differences in those who dropped out during the study and those who remained, indicating that attrition bias in this study was under control.
Selective Reporting (Reporting Bias)
As we have followed the analyze plan in the protocol accepted by the ethical board in Denmark, we believe we have reported all relevant data and not suppressed negative findings. Reporting bias should therefore be under control.
Baseline Characteristic Balanced
At baseline, the proportion of participant grated as high caries risk children was the same in the 2 groups (19%), Further, no significant differences in caries prevalence on teeth which should have fixed appliances a head in the two groups (Table 1), in plaque occurrence (Table 2), and concerning gingival status (Table 3) were noted. The p-values>0.240.
Free of Contamination/Co-Intervention
During the orthodontic treatment the children follows the normal child dental health service program in their home municipality. Caries management is a risk related program, so children who receive orthodontic treatments are covered more closely than those who do not need orthodontic treatment. Under normal considerations all participants in the present study would have been under special surveillances. However, the Corona epidemic was a major problem during the first ½ of the year 2021 in Denmark, so more less the local Child Dental Health Service offered only emergency treatment.
References
- Nørrisgaard PE, Qvist V, Ekstrand K. Prevalence, risk surfaces and inter-municipality variations in caries experience in Danish children and adolescents in 2012. Acta Odontol Scand. 2016; 74: 291-7.
- Ekstrand KR, Christiansen J, Christiansen MEC, Bakhshandeh A. The Caries Experience in the Child and Adolescents Dental Care in Denmark from 1972 to 2022. A narrative interpretation of succes. Dan Dent J. 2023; 127.
- Marinho VC, Chong LY, Worthington HV, Walsh T. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2016; 7: CD002284.
- Thylstrup A, Bruun C, Holmen L. In vivo caries models--mechanisms for caries initiation and arrestment. Adv Dent Res. 1994; 8: 144-57.
- Van der Kaaij NC, van der Veen MH, van der Kaaij MA, ten Cate JMA. A Prospective, Randomized Placebo-Controlled Clinical Trial on the Effects of a Fluoride Rinse on White Spot Lesion Development and Bleeding in Orthodontic patients. Eur J Oral Sci. 2015; 123: 186-93.
- Brøchner A, Christensen C, Kristensen B, Tranæus S, Karlsson L, Sonnesen L, et al. Treatment of post-orthodontic White spot lesions with casein phosphopeptide-stabilised amorphous calcium phosphate. Clin Oral Investig. 2011; 15: 369-73.
- Benson PE, Shah AA, Millett DT, Dyer F, Parkin N, Vine RS. Fluorides, orthodontics and demineralization: A systematic review. J Orthod. 2005; 32: 102-14.
- Sonesson M, Brechter A, Abdulraheem S, Lindman R, Twetman S. Fluoride varnish for the prevention of White spot lesions during orthodontic treatment with fixed appliances: A randomized controlled trial. Eur J Orthod. 2020; 42: 326-30.
- Petrie A, Sabin C. Medical statistics at a glance. 3rd ed. Singapore: Wiley-Blackwell. 2009.
- Løe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967; 38: 610-6.
- Ekstrand KR, Martignon S, Ricketts DJ, Qvist V. Detection and activity assessment of primary coronal caries lesions: A methodologic study. Oper Dent. 2007; 32: 225-35.
- Pitts NB, Ekstrand KR, ICDAS Foundation. International Caries Detection and Assessment System (ICDAS) and its international caries classification and Management System (ICCMS) – methods for staging of the caries process and enabling dentists to manage caries. Community Dent Oral Epidemiol. 2013; 41: e41-52.
- Ekstrand KR, Bruun G, Bruun M. Plaque and gingival status as indicators for caries progression on approximal surfaces. Caries Res. 1998; 32: 41-5.
- Pørksen CJ, Keller MK, Damholt A, Frederiksen AKS, Ekstrand KR, Markvart M, et al. The effect of a lozenge combining prebiotic arginine and probiotics on caries increment in children during 10-12 months. A randomized clinical Trial. J Dent. 2023; 135: 104599.
- Ekstrand KR, Qvist V. The Impact of a National Caries Strategy in Greenland after 4 years. Int J Paediatr Dent. 2015; 25: 255-66.
- Siegel S, Castellan NJ. Nonparametric statistics. 2nd ed. New York: McGraw-Hill Book Company. 1988.
- Tufekci E, Dixon JS, Gunsolley JC, Lindauer SJ. Prevalence of White spot lesions during orthodontic treatment with fixed appliances. Angle Orthod. 2011; 81: 206-10.
- Lucchese A, Gherlone E. Prevalence of white-spot lesions before and during orthodontic treatment with fixed appliances. Eur J Orthod. 2013; 35: 664-8.
- Twetman SHA, Ekstrand KR. Caries management by influencing minerali¬zation. In: Hendrik Meyer-Lückel H, Paris S, Ekstrand KR, editors. Caries management – science and clinical practice. Georg Thieme verlag KG. 2013; 177-90.
Citation:Ekstrand KR, Tronier-Knowlton J, Mikkjalsdóttir R, Fedders SB, Heidke R, et al. The Efficacy of Fluoride Rinse on Caries Increment, Plaque Occurrence and Gingival Status in Children Undergoing Orthodontic Treatment. A Randomized Controlled Clinical Trial with Results after 6- and 12 Months. J Dent & Oral Disord. 2023; 9(1): 1178.