
Case Report
Austin J Clin Case Rep. 2023; 10(1): 1273.
An Interesting Case of Breast Implant-Associated Anaplastic Large Cell Lymphoma
Hernandez C¹*, KC B¹, Costaldi M³, and Ghimire K²
¹Department of Medicine, State University of New York Upstate Medical University, USA
²Department of Hematology and Oncology, State University of New York Upstate Medical University, USA
³Department of Pathology, State University of New York Upstate Medical University, USA
*Corresponding author: Hernandez CDepartment of Internal Medicine State University of New York Upstate Medical University 750 E Adams St Syracuse NY 13210 USA. Phone: 718-753-0358; Email: hernanca@upstate.edu
Received: January 31, 2023; Accepted: March 06, 2023; Published: March 13, 2023
Abstract
Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) is a T-cell non-Hodgkins lymphoma that is a rare complication seen with textured breast implants. We describe the case of a 49-year-old woman with a past medical history of stage IIA infiltrating ductal carcinoma of the right breast status post bilateral mastectomy followed by chemotherapy and radiation therapy. The patient subsequently had textured implants bilaterally placed. Thirteen years later, she presented with right breast soreness with edema; pathology confirmed BIA-ALCL.
Keywords: Breast implant-associated cancer; Lymphoma; ALK-negative
Introduction
Anaplastic Large Cell Lymphoma (ALCL) is a type of T-cell non-Hodgkins lymphoma with large pleomorphic lymphoid cells with abundant cytoplasm and horseshoe-shaped nuclei positive for CD30 immunohistochemical staining [1]. The different types of ALCL are systemic and peripheral or localized. ALCL can be sub classified by the presence of the Anaplastic Lymphoma Kinase (ALK) gene or lack of the mutation designated as ALK-positive and ALK-negative, respectively. ALK-positive has the t(2;5) fusion translocation involving the ALK gene and Nucleophosmin (NPM1) gene [2].
Localized ALCL variations included Cutaneous ALCL and Breast Implant-associated Anaplastic Large Cell Lymphoma (BIA-ALCL). BIA-ALCL is very rare [3], with an incidence of 1 in 500,000 and occurring only in textured implants. BIA-ALCL is a peripheral lymphoma that typically presents in the seroma surrounding the breast implant and scar tissue. The first case of ALCL associated with textured saline breast implants was reported in 1997 [4].
In 2012, the American Society of Plastic Surgeons (ASPS), in collaboration with The Plastic Surgery Foundation (PSF) and the United States Food and Drug Administration (FDA), created The Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma etiology and Epidemiology (PROFILE) registry to gather demographic and clinical data on reported cases in the United States [3,5]. Data from the PROFILE registry shows a correlation between textured breast implants and the development of BIA-ALCL. In 2016, BIA-ALCL was recognized as a distinct sub classification of ALCL by the World Health Organization [6]. Most BIA-ALCL was encountered with the Allergan Biocell textured implants, which were recalled in 2019 [7].
The median age at diagnosis of BIA-ALCL is 52 years. The average time to onset from textured implant ranges from 7-10 years; however, cases have been reported as early as four months post-implantation [3]. Common symptoms include swelling, pain, and redness of the affected breast [8]. Rarer symptoms include lymphadenopathy, capsular contracture, and B symptoms such as fevers, night sweats, and weight loss. These symptoms are due to periprosthetic effusion in which atypical lymphoid cells are found.
Case Presentation
The patient is a 49-year-old female with a past medical history of hypertension, hyperlipidemia, anxiety, stage IIA (T1c N1 M0) ER/PR-positive, HER-2/neu negative infiltrating ductal carcinoma of the right breast diagnosed in 2009 status post bilateral with implant reconstruction. She received adjuvant Doxorubicin and Cyclophosphamide with weekly Paclitaxel and post-mastectomy radiation. She completed five years of adjuvant endocrine therapy, initially with tamoxifen for three years, followed by exemestane (6-methylenandrosta-1, 4-diene-3,17-dione) after a bilateral salpingo-oophorectomy. She completed adjuvant endocrine therapy in 2014, four months prior to the 5-year mark, due to arthralgias and weight gain.
However, eight years after completing endocrine therapy, she reported increasing swelling and fullness of her right breast, together with some discomfort. The patient had no fevers, chills, diaphoresis, or weight loss on the physical exam. There was concern that she could have capsular contracture with possible infection. Ultrasound showed a moderate to large amount of fluid with scattered thin septations, which were seen around the right breast implant. She underwent ultrasound-guided aspiration of 110 ml of fluid and was placed on antibiotics. Aspiration of the right breast demonstrated atypical cell morphology.
A subsequent MRI of the breast was notable for a moderate right-sided implant fluid collection with asymmetric mild, right capsular thickening with adjacent fat stranding and edema, possible etiology of inflammation, infection, or neoplasm. There was no enhancement in the left breast or axillary adenopathy.
The patient had a capsulectomy a month later with improvement in symptoms. The right breast periprosthetic capsule showed dense fibrous tissue, but no discrete masses were noted. The treatment was the removal of the implant. Since the disease was limited to the effusion and early capsule infiltration, it was staged IB (T2N0M0).
Cytology from the right breast seroma was positive for malignant cells. The specimen contained malignant cells with a small to moderate amount of cytoplasm and markedly atypical malignant cells with partially vacuolated cytoplasm and enlarged and irregular nuclei with prominent nucleoli along with the occasional "hallmark" cells (Figure 4).
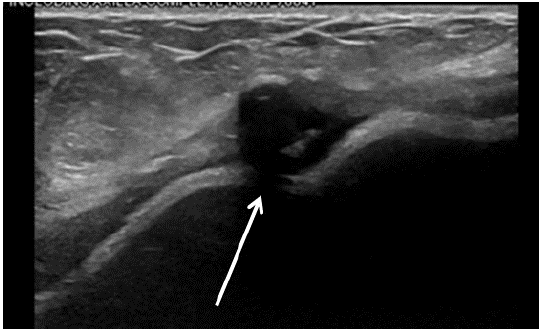
Figure 1: Ultrasound of the fluid surrounding the right breast. There was a moderate to large amount of fluid with scattered thin septations seen about the right breast implant. Area of likely fat necrosis is on the breast at 9 o’clock position, 12 cm from the nipple.
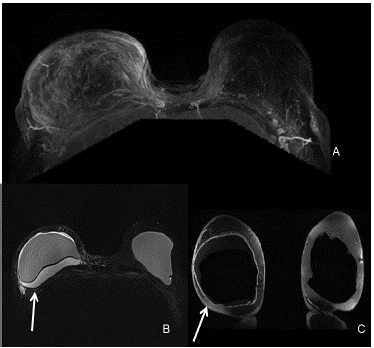
Figure 2: MRI of bilateral breasts. (A) The right breast shows moderate right sided fluid collection. There is asymmetric mild right capsular thickening with adjacent fat stranding and edema. No suspicious enhancement was seen in the left breast. (B) The silicone only images are STIR0 images in which fat is suppressed, combined with water suppression so the only signal seen is the silicone material which is the best for studying rupture of the envelope and detecting silicone outside of the envelope or capsule. (C) The water only image is a combination of fat saturation and silicone suppression which gives information about the fluid collections within or around the breast implant. When the contour of the implant is more rounded, it is an indication of capsular contraction. The degree of contractions is done clinically.
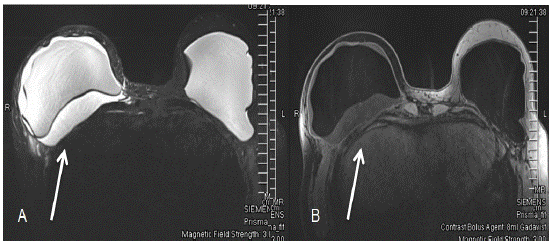
Figure 3: Bilateral breast MRI with and without contrast showing fluid surrounding right breast implant. (A) The first image shows the silicone-only image. (B) The second image shows only water.
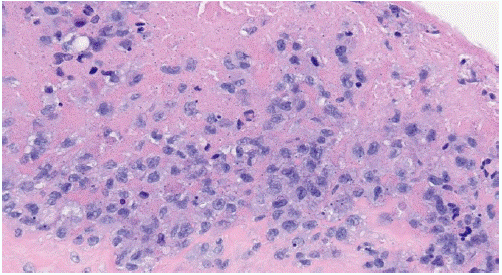
Figure 4: Anaplastic Large Cell Lymphoma H&E stain (40x) Breast Implant Capsule. The image shows “hallmark cells” which have abnormal kidney-shaped or horseshoe-shaped nuclei in anaplastic large cell lymphoma.
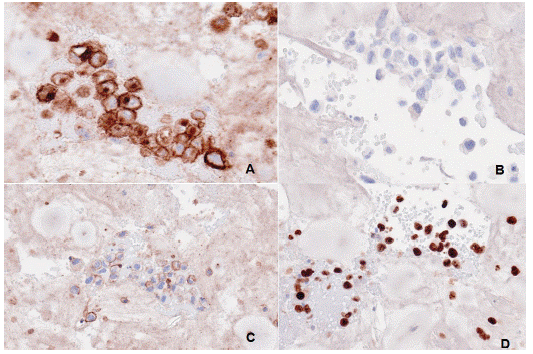
Figure 5: Immunohistochemistry of seroma fluids. (A) The image shows CD30 expression (B) no Anaplastic Lymphoma Kinase (ALK) protein indicative of ALK Negative type of Anaplastic Large Cell Lymphoma. (C) It was positive for CD45 (D) and positive for Ki67 on cell block of the seroma fluid.
Immunohistochemical stains showed that the malignant cells were positive for CD45 and CD30. A CD3 stain showed patchy and weak positively in the malignant cells. A CD20 stain was negative. Stains for PAX5, ALK1, and cytokeratin AE1/AE3 were also negative. A Ki-67 stain showed a higher proliferative index within the malignant cells more than 90%. These findings were consistent with BIA-ALCL.
A Positron Emission Tomography (PET) scan a month after the capsulectomy showed bilateral small chest wall seromas with surrounding soft tissue density with increased metabolic activity. PET activity in both chest wall seromas was likely due to the recent surgery. However, local recurrence could not be ruled out. Follow-up PET scan (three months after capsulectomy) showed resolution of previously noted seromas with significantly improved bilateral metabolic activity with residual bilateral metabolic activity. Of note, the activity was more diffuse and current scans show a more focal appearance. Currently, the patient is on active surveillance with follow-up imaging every six months and planned for two years.
Discussion
ALCL is a group of T-cell non-Hodgkin's lymphomas (systemic or peripheral) that universally express CD30 Ki-1 antigen [9,10]. ALCL can also be associated with translocations involving the Anaplastic Lymphoma Kinase (ALK) gene ALK-negative is the more indolent primary cutaneous ALCL, as seen in our case. A Ki-67 stain showed an increase in proliferative index greater than 90%. Ki-67 is a cellular proliferation marker used for cancer prognosis and to estimate treatment response.
BIA-ALCL is a novel manifestation of the site and material-specific lymphoma and a recently recognized complication of textured breast implants [11]. It may form a locally invasive mass and metastasize to regional lymph nodes or distant sites [3]. Staging of BIA-ALCL follows the MD Anderson Solid Tumor Staging System modeled after the American Joint Committee on Cancer TNM stages. Patients have a spectrum of disease from IA only involving the effusion, IB, 1C, IIA, IIB, and IV [12]. The patient had early capsule infiltration, no lymph node involvement, and no distant spread with a Stage of IB.
Although the pathophysiology of BIA-ALCL is still unknown, recent studies suggest that chronic inflammation caused by a bacterial biofilm of textured implants may mediate T-cell hyperplasia and the development of ALCL. The development of BIA-ALCL is less common in smooth implants [13]. A fibrous capsule forms after the placement of the breast implant. It is a primarily T-cell-driven inflammatory response. BIA-ALCL forms a malignant effusion between the breast implant and the surrounding capsule. With progression, the neoplastic cells merge into a mass that can invade the capsule into the neighboring tissues. CD30+ clonal T-cells have been identified within the capsule of a benign late-seroma, further signifying progression from benign lymphoproliferative disorder to BIA-ALCL [3,9].
In the case study by George et al., the indolent tumor transformed into an aggressive ALCL with evidence of in vivo cytogenetic evolution after capsulectomy without chemotherapy in a six-month span. The lymph node tissue expressed CD30 and showed rearranged TCR genes that were ALK-negative and showed complex cytogenetic abnormalities suggesting genetic instability places a large role in BIA-ALCL pathogenesis or tumor progression [14-17].
In 2016, the National Comprehensive Cancer Network (NCCN) [18] published guidelines for diagnosing and treating BIA-ALCL. These guidelines were updated in 2019 and stated that following a diagnosis, complete resection of the implants, lymphoma, and any surrounding fibrous capsule should be performed. The current evidence-based algorithm for achieving a diagnosis includes obtaining a biopsy, cytology, flow cytometry for T cell clone, and Immunohistochemistry (IHC) for CD30. The next step is surgery, staging, and starting systemic treatment, such as cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). The treatment plan must also consider other factors, such as the patient's comorbidities and goals of care. Furthermore, any late-onset unilateral effusions or masses should be punctured and analyzed. Usually, a PET scan is recommended for staging and surgical planning.
Of treatments available for BIA-ALCL, surgery provides the most significant benefit in terms of survival and prevention of recurrence [3]. Most patients present with stage I. Surgical treatment can consist of capsulectomy or mastectomy with excision of the mass. In our case, removal of the implants yielded good results since there were no discrete masses or extension noted.
In cases of local invasion or unresectable disease, radiation therapy may also be considered [18]. Adjuvant CHOP chemotherapy and radiation can also be done. With limited available clinic guidance, previous case reports have described the use of anti-CD30 agents, such as brentuximab vedotin, as monotherapy or in combination with other agents [19].
The CD30-targeting immunoconjugate, brentuximab vedotin, has been used along with chemotherapy [3]. The ECHELON-2 study [19] showed brentuximab vedotin plus cyclophosphamide, doxorubicin, and prednisone (A+CHP) had better overall survival than CHOP in patients with CD30-Positive Peripheral T-Cell Lymphomas (PTCL). A five-year update of ECHELON-2 of patients with PTCL with A+CHP continued to show better survival than CHOP therapy [20].
BIA-ALCL has a good prognosis with an overall five-year survival rate of 89-92%. In systemic ALCL, there is a five-year survival rate of 37-49% in ALK-negative and 70-93% in ALK-positive. However, patients with masses with extension beyond the implant capsule have a worse prognosis. Systemic treatment can be considered in patients with Lugano classification staging of lymphomas [21] with stage II-IV disease [15]. After completing treatment, the patients should be monitored with surveillance every three to six months for two years and then as needed. Based on the ECHELON-2 study in patients with PTCL, A-CHP has shown good survival benefit compared to CHOP and should be considered for future treatment of patients with BIA-ALCL.
Conclusion
BIA-ALCL is a sporadic type of lymphoma and an emerging complication of breast implantation. Although more cases have been identified over the years, leading to the development of new guidelines, there remains no standard of care for this disease, and data are limited to case reports. Treatment requires a multidisciplinary approach.
References
- Shustov A, Soma L. Anaplastic Large Cell Lymphoma: Contemporary Concepts and Optimal Management. Cancer Treat Res. 2019; 176: 127-44.
- Kaseb H, Mukkamalla SKR, Rajasurya V. Anaplastic Large Cell Lymphoma. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC. 2022.
- Stack A, Ali N, Khan N. Breast Implant-associated Anaplastic Large Cell Lymphoma: A Review with Emphasis on the Role of Brentuximab Vedotin. J Cell Immunol. 2020; 2: 80-9.
- Keech JA, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997; 100: 554-5.
- McCarthy CM, Loyo-Berríos N, Qureshi AA, Mullen E, Gordillo G, et al. Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology (PROFILE): Initial Report of Findings, 2012-2018. Plast Reconstr Surg. 2019; 143: 65s-73s.
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127: 2375-90.
- Danino MA, Dao L, Retchkiman M, Matetsa E, Iezzoni J, et al. Analysis of Allergan’s Biocell Implant Recall in a Major University Breast Center. Plast Reconstr Surg Glob Open. 2020; 8: e2906.
- Berlin E, Singh K, Mills C, Shapira I, Bakst RL, et al. Breast Implant-Associated Anaplastic Large Cell Lymphoma: Case Report and Review of the Literature. Case Rep Hematol. 2018; 2018: 2414278.
- Mehta-Shah N, Clemens MW, Horwitz SM. How I treat breast implant–associated anaplastic large cell lymphoma. Blood. 2018; 132: 1889-98.
- Stein H, Mason DY, Gerdes J, O’Connor N, Wainscoat J, et al. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985; 66: 848-58.
- Brody GS, Deapen D, Taylor CR, Pinter-Brown L, House-Lightner SR, et al. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg. 2015; 135: 695-705.
- Clemens MW, Medeiros LJ, Butler CE, Hunt KK, Fanale MA, et al. Complete Surgical Excision Is Essential for the Management of Patients With Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J Clin Oncol. 2016; 34: 160-8.
- Barr SP, Hill EW, Bayat A. Novel Proteomic Assay of Breast Implants Reveals Proteins With Significant Binding Differences: Implications for Surface Coating and Biocompatibility. Aesthet Surg J. 2018; 38: 962-9.
- George EV, Pharm J, Houston C, Al-Quran S, Brian G, et al. Breast implant-associated ALK-negative anaplastic large cell lymphoma: a case report and discussion of possible pathogenesis. Int J Clin Exp Pathol. 2013; 6: 1631-42.
- Hallberg B, Palmer RH. The role of the ALK receptor in cancer biology. Ann Oncol. 2016; 27: iii4-iii15.
- Lazzeri D, Agostini T, Bocci G, Giannotti G, Fanelli G, et al. ALK-1–Negative Anaplastic Large Cell Lymphoma Associated With Breast Implants: A New Clinical Entity. Clinical Breast Cancer. 2011; 11: 283-96.
- Tabbó F, Barreca A, Piva R, Inghirami G. ALK Signaling and Target Therapy in Anaplastic Large Cell Lymphoma. Front Oncol. 2012; 2: 41.
- Clemens MW, Miranda RN. Commentary on: Lymphomas Associated With Breast Implants: A Review of the Literature. Aesthet Surg J. 2015; 35: 545-7.
- Horwitz S, O’Connor OA, Pro B, Illidge T, Fanale M, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019; 393: 229-40.
- Horwitz S, O’Connor OA, Pro B, Trümper L, Iyer S, et al. The ECHELON-2 Trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann Oncol. 2022; 33: 288-98.
- Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014; 32: 3059-68.