
Research Article
Austin Cell Biol. 2015; 1(1): 1003.
Influence of Ferula Hermonis Acetonic Extract on Exercise Adapted Rat Gastrocnemius
Allouh MZ* and Said RS
Department of Anatomy, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan
*Corresponding author: Mohammed Z Allouh, Department of Anatomy, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan
Received: December 17, 2015; Accepted: December 30, 2015; Published: December 31, 2015
Abstract
Ferula hermonis boiss is a medicinal plant that grows in the Mediterranean region. It has been reported that treatment with acetonic extract from the root of this plant improves the growth of tibialis anterior muscle fibers when associated with exercise. This study investigated the effects of F. hermonis extract alone or combined with exercise on the morphology of rat Gastrocnemius (GC) muscle fibers. Adult male rats were divided into four groups: Control Sedentary (CS) that had no treatment or exercise; Ferula Sedentary (FS) that was orally treated with ferula extract at a dose of 60 mg/kg every other day over a period of 20 days; Control Exercised (CE) that was trained by swimming for 40 minutes every other day; and Ferula Exercised (FE) that received ferula and performed exercise. At the end of experiments, the fiber diameter, number of muscle nuclei, nuclear length, and nuclear area of GC were measured by using immune fluorescent techniques and software analyses. FE group showed significant increases in nuclear area and nuclear number compared to the other groups. In addition, the fiber diameter increased significantly in FE group compared to CS group. In conclusion, short term exercise combined with administration of F. hermonis extract was more effective in enhancing the growth of GC muscle fibers than exercise alone.
Keywords: Ferula; Skeletal muscle fiber; Muscle nuclei; Testosterone
Abbreviations
CS: Control Sedentary; CE: Control Exercised; FS: Ferula Sedentary; FE: Ferula Exercised; GC: Gastrocnemius
Introduction
Ferula hermonis (Boiss.) is a medicinal plant that belongs to the apiaceae family and grows abundantly in the Middle East [1,2]. The root extract of this plant is commonly used as a treatment for sexual impotence. Recently, the root extract has been commercialized and is widely used among Middle Eastern populations to improve performance and increase energy [3]. Several phytochemical and pharmaceutical studies were conducted on this plant to identify its active ingredients and their respective mechanism of action [4,5]. The main ingredients are four aromatic esters of sesquiterpene alcohol known as ferutin in (ferutinolp hydroxyl benzoate), teferd in (ferutinol benzoate), tefer in (ferutinolvanillate) and epoxybenz (epoxyferutinol benzoate) [4]. In addition to these esters, several naturally occurring vitamins and minerals were found in the root of F. hermonis [1].
In a previous study we reported that oral administration of acetonic extract from F. hermonis can increase serum testosterone levels and augment the effect of exercise on rat tibialis anterior muscle [3]. The rat tibialis anterior muscle is predominantly composed of glycolytic type of fibers (type IIb) [6,7]. This study aimed to investigate the reproducibility of the effect of acetonic root extract of F. hermonis on Gastrocnemius (GC) deep muscle fibers, since this portion of the muscle is composed of different fiber types (type I and type IID) [7,8]. The study tested the hypothesis that supplementation of F. hermonis extract combined with short term exercise is more effective in improving GC muscle fiber growth than exercise alone.
Materials and Methods
Experimental model
Adult male albino rats of Sprague Dawleys train, weighing about 300 g, were produced and raised in the Animal House Unit at Jordan University of Science and Technology (JUST) for this study. All animal care procedures and treatments were conducted with the approval of the Animal Care Unit Committee at JUST, and according to the guidelines of the National Institute of Health on the use and care of laboratory animals (USA).
The rats were divided randomly into four groups: Control Sedentary (CS) that had no treatment or exercise, ferula sedentary (FS) that was orally treated with ferula extract, Control Exercised (CE) that was trained by swimming, and ferula exercised (FE) that received combined treatment and exercise. Each group contained three male rats. The animals were kept under controlled temperature of 21 ±1°C with a schedule of 12 hours light and 12 hours dark (lights on 06.00–18.00 hr). Food and water were available ad libitum. The rats were given a two week adaptation period before starting the treatment.
Extract preparation and animal treatments
F. hermonis roots were obtained from a local market in Jordan and further identified by specialized botanists in The Department of Biological Sciences at Yarmouk University, Jordan. The roots were ground into powder and the acetonic extract was prepared using a Soxhlet apparatus. The extract was then filtered and the solvent from the filtrate was removed by distillation. The remaining solid extract was preserved in a refrigerator until treatment.
Just before treatment, the extract was solubilized in Tween 80 (10%) and distilled water (90%) to facilitate its oral administration to the animals in the ferula treated groups at a dose of 60 mg/kg body weight. This dose was similar to the one administered in our previous study [3]. The dose was given every other day using animal feeding intubation needles (Popper & Sons, NY, USA). Each animal received ten doses over a period of twenty days. Animals in the control groups received the same volume of vehicle media only.
Swimming exercise
Rats in the exercised groups were trained to swim in a rectangular water tank (1.5 x 1.0 m) with a water depth of40 cm and a temperature of approximately 34 36°C. Swimming was selected as the mode of exercise because it is considered to be less traumatic for the animals and does not cause foot injures. The swimming protocol was conducted in two phases: adaptation and training. The adaptation phase started in the last five days of the acclimatization period, before the treatments began. The rats were exposed for 10 min swimming on the first day of adaptation. The swimming period was extended by 10 minutes every day until the rats were swimming for a total of 40 min. On the final day of the adaptation phase, the animals were allowed to rest. The training phase consisted of swimming for 40 min on the same day of ferula treatment, 45 min after receiving the oral dose. The rats were trained 10 sessions over a period of 20 days. All swimming sessions were supervised to avoid floating and/or clinging of the rats.
Testosterone assay
The rats were euthanized by diethyl ether and trunk blood was collected into centrifuge tubes. Serum was prepared by centrifugation at 3000 rpm for 30 min, and then stored frozen (40°C) until testosterone assay. The testosterone concentration was determined by using the Testosterone Enzyme Immunoassay test kit (Bio Check Inc., CA, USA). The minimum detectable concentration of this assay was estimated to be 0.05 ng/ml and cross reactivity with other corticosteroids was minimal (<0.05%).
Tissue preparation and sectioning
Muscle samples were excised from the deep portion of the GC muscle of each rat. The samples were cut parallel to the orientation of the muscle fibers. Each sample was then coated with Optimal Cutting Temperature (O.C.T.) compound (Bio Optica, Milano, Italy) and immediately frozen in 2-methylbutane cooled via liquid nitrogen. Samples were then stored at 40°C until sectioning.
Serial cross-sections of 10 μm thickness were cut at -20°C using a cryostat. Every two serial sections were picked up on a Super frost Plus microscopic slide (Santa Cruz Biotechnology Inc., CA, USA). The reason for collecting two sections on each slide was to increase the possibility of choosing better fields for imaging. Serial slides bearing sections were numbered and stored at -20°C.
Antibodies
The primary antibody used for immune staining was antilaminin (Sigma Chemical Co., MI, USA). Anti laminin is a rabbit polyclonal antibody that was developed against the glycoprotein laminin of mouse origin and was used to detect the basal laminae of skeletal muscle fibers at a dilution of 1:200. Tetra methyl rhodamine secondary antibody (Sigma Chemical Co.) was used to label antilaminin in red when viewed with an epifluorescent microscope. The secondary antibody was prepared in phosphate-buffered saline (PBS) at a dilution of 1:400.
Immunocytochemical protocol
Slides were removed from the -40°C and air dried for 15 min. Sections were then treated for 30 min with 200 μl of blocking solution, which consisted of 5mM Ethylene Diamine Tetra Acetic Acid (EDTA) in phosphate-buffered saline (PBS), 5% goat serum and 1% bovine serum albumin. After that, blocking solution was drained from each slide and anti-laminin primary antibody diluted in blocking solution was added to each slide (150μl) and incubated over night at 4°C. Slides were then washed three times in fresh PBS solution for 5 min per wash. The secondary anti body was then applied over the sections on each slide for 40 min at room temperature, followed by two 5 min washes in fresh PBS. Finally, slides were mounted with cover slips using Ultra Cruz mounting medium containing DAPI (Santa Cruz Biotechnology Inc.) and sealed before being examined under epifluorescent microscopy.
Image analysis and data collection
Four different fields of view were captured from each immune fluorescent slide using a fluorescent microscope equipped with a digital camera. Two epifluorescent images, each viewed through a different wavelength filter, were acquired from each field. Images were subsequently transferred to a computer, and the two images of each field of view were superimposed using Adobe Photoshop program (Adobe System Inc., CA, USA). The resultant immune fluorescent images showed all nuclei in blue and basal laminae in red (Figure 1).
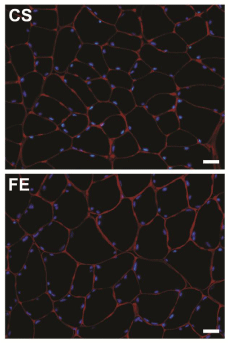
Figure 1: Immunocytochemical labeling of cross sections from rat
gastrocnemius muscle. Muscles were from two different groups; Control
Sedentary (CS), and Ferula Exercised (FE). In both images, laminin is
colored in red and all nuclei in blue. Scale bars= 50 μm.
The ellipse minor axis (shorter diameter of an ellipse structure) was used to assess fiber size. Minor axes of 400 contiguous fiber cross sections were measured from each rat. The basal laminae images were used to measure the ellipse minor axis of individual fibers by using Image J program (developed by US National Institute of Health and available on the internet, Version 1.48v).
The number of all muscle nuclei beneath the basal lamina was counted for each of the 400 fibers. After that, the number of nuclei per unit length of fiber was calculated for each rat using the formula demonstrated by Castillo de Maruenda and Franzini-Armstrong [9]. This formula is: N= A/(Ln + M) × U, where N is the number of cells per unit length of fiber, A is the mean number of nuclei per fiber cross section, Ln is the average length of the nucleus, M is the thickness of tissue section and U is the unit length of fiber (1 mm).
The length and area of muscle nuclei were measured from longitudinal sections of 10 μm thickness cut at -20°C using a cryostat (Leica, CM3050 S). Applying the same immune cytochemical protocol described above, we were able to obtain longitudinal images for GC muscle from different rats in each group (Figure 2). After that, the measurements of 50 consecutive nuclei from each rat were measured using Image J.
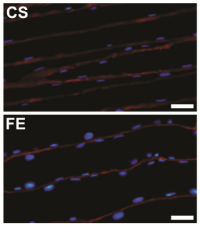
Figure 2: Immunocytochemical labeling of longitudinal sections from rat
gastrocnemius muscle. Muscles were from two different groups; Control
Sedentary (CS), and Ferula Exercised (FE). In both images, laminin is
colored in red and all nuclei in blue. Scale bars= 50 μm.
Statistical analysis
The samples were blocked into four groups. Levene’s test for homogeneity of variance was first applied. After determining the homogeneity of variance, data was evaluated by one way analysis of variance (ANOVA) at 5% and 1% levels of significance. If a significant difference was detected, Fisher’s least significant difference test was performed for post hoc analysis.
Results
Testosterone serum level
Serum testosterone level increased significantly (P<0.05) in FE rats compared to CS rats (Figure 3). Serum testosterone in FE group was 3.79 ± 0.16 ng/ml while in CS group it was 2.64 ± 0.34 ng/ml (mean ± SE).

Figure 3: Influence of Ferula hermonisacetonic root extract with exercise on
serum testosterone level in adult male rats for four different groups (CS =
Control Sedentary, FS = Ferula Sedentary, CE = Control Exercised, FE =
Ferula Exercised). Each value represents the mean ± standard error (SE).
Different letters indicate significantly different values. *P<0.05 (ANOVA, LSD
post hoc).
Fiber Morphology
GC muscle fibers were significantly (P<0.05) larger in the FE group compared to the CS group. Though, there was no significant difference (P>0.05) in fiber size in CE or FS groups in comparison to CS group. The mean diameter in FE group was about 13% larger than the mean diameter of CS group (Table 1).
Group
Fiber Diameter
(µm)
Number of nuclei per Fiber Cross Section
Number of nuclei per mm of Fiber
Sarcoplasmic volume per nucleus
(µm3 × 103)
CS
73.07 ± 3.20
2.68 ± 0.09
93.72 ± 3.20
44.78 ± 2.87
FS
74.43 ± 2.33
2.51 ± 0.17
87.67 ± 5.97
50.38 ± 5.82
CE
75.97 ± 2.35
2.62 ± 0.28
91.51 ± 9.88
50.40 ± 4.22
FE
82.42 ± 2.98*
3.81 ± 0.49è
133.19 ± 17.00è
40.88 ± 3.03
Values are representing the mean ± standard error for four different groups (CS = control sedentary, FS = ferula sedentary, CE = control exercised, FE = ferula exercised). *P<0.05, significantly differs from CS group only (ANOVA, LSD post hoc). *P<0.05, significantly differs from all groups (ANOVA, LSD post hoc).
Table 1: Influence of Ferula hermonis acetonic root extract with short term exercise on the morphology of skeletal muscle fibers within gastrocnemius of adult male rat.
In addition, the number of muscle nuclei per mm of fiber length was significantly (P<0.05) greater in FE group compared to the other three groups. No significant increase (P>0.05) in the number of nuclei per mm of fiber was found among CE, FS and CS groups. There was on average about 39 more nuclei per mm in GC fibers from FE group in comparison to the CS group (Table 1).
Nuclear Measurements
There were no significant differences (P>0.05) in the mean lengths of muscle nuclei between the groups. The overall mean nuclear length for each group is shown in (Table 2). However, a significantly greater nuclear area was found in FS (P<0.05) and CE (P<0.01) groups compared to CS group, and in FE (P<0.01) group compared to the other three groups (Table 2).
Group
nuclear Length (mm)
nuclear Area (mm2)
CS
18.63 ± 0.52
95.65 ± 6.06a
FS
19.57 ± 0.68
116.02 ± 6.05b*
CE
19.98 ± 0.91
126.55 ± 6.20b**
FE
18.36 ± 0.74
181.77 ± 7.87c**
Values are representing the mean ± standard error for four different groups (CS = control sedentary, FS = ferula sedentary, CE = control exercised, FE = ferula exercised). Different letters indicate significantly different values. *P<0.05, **P<0.01 (ANOVA, LSD post hoc).
Table 2: Influence of Ferula hermonis acetonic root extract with short term exercise on the morphology of muscle nuclei within gastrocnemius of adult male rat.
Discussion
The results of this study showed a significant increase in the total number of nuclei in the FE group when compared to the other three groups; this increase might be attributed to the greater anabolic activity within the GC of FE group. This prospect is further supported by the significant increase in nuclear area in GC fibers of FE group compared to other groups. In addition, rats in FE group showed hypertrophy in their GC muscles, indicated by the significant increase in their fiber size compared to CS group. However, rats in the FS and CE groups didn’t show any significant changes in their fiber morphology.
The exact mechanism by which ferula extract can cause this enhancement is not fully understood, one of the possible pathways might involve the significant increase in serum testosterone due to ferula administration. In addition to F. hermonis, several other medicinal plants have been used to induce testosterone secretion, including the Orchisanatolica (orchids) [10], and Cyperus esculentus (Tiger Nut) [11]. These herbs are believed to contain several compounds that are proven to stimulate testosterone production. The phytoestrogen components in F. hermonis including ferutinin and teferdin are the most anticipated compounds to increase serum testosterone [3]. Their speculated mechanisms of action include; increasing the secretion of thyroid hormones [12], which are known to increase the synthetic activity of Leydig cells [13], another mechanism involves the activation of hypothalamus-pituitary-testicular axis through the production of nitric oxide in the hypothalamus [14,15].
The frequency of administration and the chemical nature of the solvents used in the extraction process may play an important role in determining the effect of ferula supplementation. In fact, it has been reported that daily administration of acetonic extract for 10 days leads to significant reduction in serum testosterone [16], while intermittent, every other day, administration induced a significant boost in testosterone concentration [3]. In addition, opposing outcomes were reported by using different extracts of F. hermonis. For example, rats which received aqueous extract showed testicular atrophy and reduction in weight gain, suggesting an anti androgenic effect for the aqueous extract of F. hermonis [17].
Conclusion
This study demonstrated that the combined effect of the F. hermonisacetonic extract with short term exercise is more effective than exercise alone in inducing the growth of rat GC muscle. The statistical analysis showed that this effect of ferula extract is additive to exercise rather than synergistic. Future investigations are warranted to validate the effectiveness of this herb and its useful application in human muscle therapies.
Acknowledgement
Funds for this study were provided by a grant to MZA from the Deanship of Research at JUST.
References
- Naguib YM. Pharmaceutically active composition extracted from Ferula hermonis and process of its extraction. United States Patent 6623768, 2003.
- Auzi AA, Gray AI, Salem MM, Badwan AA, Sarker SD. Feruhermonins A-C: three daucane esters from the seeds of Ferula hermonis (Apiaceae). J Asian Nat Prod Res. 2008; 10: 711-717.
- Allouh MZ. Effect of Ferula hermonis root extract on rat skeletal muscle adaptation to exercise. Exp Biol Med (Maywood). 2011; 236: 1373-1378.
- Abourashed EA, Galal AM, El-Feraly FS, Khan IA. Separation and quantification of the major daucane esters of Ferula hermonis by HPLC. Planta Med. 2001; 67: 681-682.
- Diab Y, Dolmazon R, Bessie`re JM. Daucane aryl esters composition from the Lebanese Ferula hermonisBoiss (zallooh root). FlavourFragr J. 2001; 16: 120-122.
- Hämäläinen N, Pette D. The histochemical profiles of fast fiber types IIB, IID, and IIA in skeletal muscles of mouse, rat, and rabbit. J Histochem Cytochem. 1993; 41: 733-743.
- Staron RS, Kraemer WJ, Hikida RS, Fry AC, Murray JD, Campos GE. Fiber type composition of four hindlimb muscles of adult Fisher 344 rats. Histochem Cell Biol. 1999; 111: 117-123.
- Cornachione AS, Benedini-Elias PC, Polizello JC, Carvalho LC, Mattiello-Sverzut AC. Characterization of fiber types in different muscles of the hindlimb in female weanling and adult Wistar rats. Acta Histochem Cytochem. 2011; 44: 43-50.
- Castillo de Maruenda E, Franzini-Armstrong C. Satellite and invasive cells in frog sartorius muscle. Tissue Cell. 1978; 10: 749 772.
- Allouh MZ, Khouri NA, Daradka HM, Kaddumi EG. Orchisanatolica root ingestion improves sexual motivation and performance in male rats. J Complement Integr Med. 2010; 7: 39.
- Allouh MZ, Daradka HM, Ghaida JH. Influence of Cyperus esculentus tubers (Tiger Nut) on male rat copulatory behavior. BMC Complement Altern Med. 2015; 15: 331.
- Gunnarsson D, Selstam G, Ridderstråle Y, Holm L, Ekstedt E, Madej A. Effects of dietary phytoestrogens on plasma testosterone and triiodothyronine (T3) levels in male goat kids. Acta Vet Scand. 2009; 51: 51.
- Wagner MS, Wajner SM, Maia AL. The Role of Thyroid Hormone in Testicular Development and Function. J Endocrinol. 2008; 199: 351-365.
- Colman-Saizarbitoria T, Boutros P, Amesty A, Bahsas A, Mathison Y, GarridoMdel R, et al., Ferutinin stimulates nitric oxide synthase activity in median eminence of the rat. J Ethnopharmacol. 2006; 106: 327-332.
- Prevot V, Croix D, Rialas CM, Poulain P, Fricchione GL, Stefano GB, et al., Estradiol coupling to endothelial nitric oxide stimulates gonadotropin-releasing hormone release from rat median eminence via a membrane receptor. Endocrinology. 1999; 140: 652-659.
- Zanoli P, Benelli A, Rivasi M, Baraldi C, Vezzalini F, Baraldi M. Opposite effect of acute and subchronic treatments with Ferula hermonis on copulatory behavior of male rats. Int J Impot Res. 2003; 15: 450-455.
- Khleifat K, Homady MH, Tarawneh KA, Shakhanbeh J. Effect of Ferula hermonis extract on social aggression fertility and some physiological parameters in prepubertal male mice. Endocr J. 2001; 48: 473-482.