
Case Report
Austin Cardio & Cardiovasc Case Rep. 2023; 8(1): 1052.
Inappropriate Shock Due to Subcutaneous Lead Body Fracture: Extraction and Reimplantation
C Moïni1,2,4; D Rahim2; N Belfeki3; A Lefoulon1; D Poindron4; N Lellouche5
1Department of Cardiology, GHSIF, Melun, France
2Department of Cardiology, Antony Private Hospital, France
3Department of Internal Medicine, GHSIF, Melun, France
4Department of Cardiology, Les Fontaines Clinic, Melun, France
5Department of Cardiology, Henri Mondor University Hospital, Creteil, France
*Corresponding author: Cyrus Moini Department of Cardiology, GHSIF, Les Fontaines Clinic, Melun and Antony Private Hospital, France. Email: cyrus.moini@ghsif.fr
Received: March 21, 2023 Accepted: April 26, 2023 Published: May 03, 2023
Abstract
Subcutaneous Implantable Cardioverter Defibrillator (S-ICD) has been developed as an alternative to Transvenous (TV) devices to reduce lead-related complications leaving the heart and vasculature untouched.
Recently, Boston Scientific published a safety notice in December 2020 regarding a subcutaneous electrode risk of fracture. In the following case report, we describe a case report experiencing this complication and present how we proceeded for lead extraction and reimplantation. The procedure remained safe and easy.
Keywords: Lead fracture; Subcutaneous ICD; Extraction
Introduction
The Implantable Cardioverter Defibrillator (ICD) has proven to be an effective strategy for sudden cardiac death prevention in patients with life-threatening ventricular arrhythmia. However, the Transvenous (TV) lead remains the Achilles’ heel of classical ICD systems (conductor fracture, insulation abrasion, extraction risk, infection …). TV lead-related complications could reach 20% in 10-year-old leads in young and active patients [1]. The S-ICD has been developed in response to these concerns.
However, Boston Scientific recently published a safety notice in December 2020 regarding a risk of body lead fracture of the subcutaneous electrode [2] (FDA class I recall). To date, 68 cases of lead fracture have been reported worldwide with an evaluated occurrence around 0.30% at 66 months [3]. According to the information provided by the company and the literature [4,5], fractures are located immediately after the distal part of the proximal electrode. This specific area is filled with adhesive during assembly and allows the connection of the conductor to the proximal sensing electrode. Fractures are described by strictly similar primary and secondary vectors; flat alternate vector; system impedance greater than 400 ohms causing beeping tones of the ICD.
If the vector is programmed to secondary or alternate sensing, warning/precursor signals of the fracture might be stored, showing non physiological mechanical artifact.
Case
In July 2020, a 58-year-old man with an ischemic cardiomyopathy was implanted with an S-ICD in secondary prevention. 23 months later, the S-ICD delivered a therapy at night and the patient was called for follow-up. The examination revealed a high electrode impedance (>400 ohms) and 3 episodes classified as “ventricular tachycardia” of which one was treated.
The 3 events showed mechanical artifacts. S-ECG control reveals identical primary and secondary vectors and a flat alternate vector (Figure 1). Chest X-ray with front and left profile confirmed the lead rupture at the left xypho-sternal angle as described in the literature. The patient was asymptomatic and did not report any chest trauma.
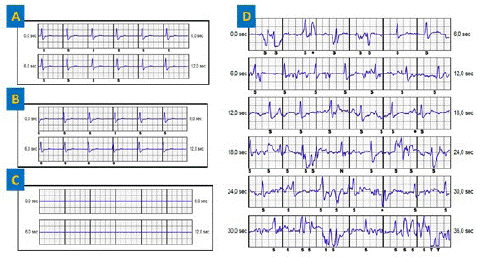
Figure 1: (A) Primary vector. (B) Secondary vector, similar to the primary vector. (C) Flat alternate vector (D) event classified as VT showing mechanical artifacts.
With a xyphoid incision, we were able to visualize a clear fracture immediately after the distal part of the proximal electrode. The proximal part of the lead was removed by simple traction without difficulties.
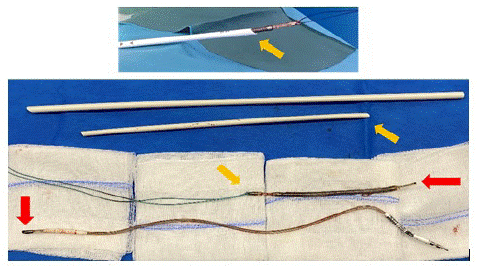
Figure 2: Top: extracted lead with the dilator sheath through distal incision.
Bottom: Lead extracted in 2 pieces thanks to the dilator sheath.
Red arrows indicate the fracture point. In orange, the direction of extraction.
To avoid partial extraction of the remaining part, in particular unwinding of the coil [5], an additional incision in distality of the lead was made. A Cook dilator sheath (ref. G19860, internal diameter 11,5Fr, length 38cm) was used; this was adjusted to the remaining length of the lead. A solid thread was inserted into the hole at the end of the lead and then mounted in the extraction sheath, which in turn was inserted through the distal incision. The lead was freed from all adhesions by making push-pull between the lead and the sheath, supplemented by clockwise and counter clockwise rotations of the sheath.
The detection parameters of the previous electrode were deemed appropriate. Therefore, the new device was also implanted left parasternal side. The additional distal incision made to extract the lead was closed to proceed to a classic 2-incisions implant technique. A new tunneling was carried out in parallel to the sternum, 1cm left away from the initial lead to avoid any floating of the lead (Figure 3). The system was tested with success (65J, 74ohms, time to treatment 17s). The control X-ray shows good positioning of the device.
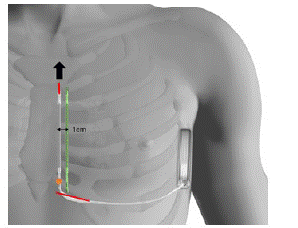
Figure 3: ●: Fracture point of the lead, distally to the proximal electrode
In red: The 2 incisions made
Arrow: Direction of extraction, through the supplementary distal incision
In green: New tunneling 1cm away from the initial lead
Discussion
It seems that the first lead incomplete S-ICD lead fracture occurred in 2020 and was not related to the recall as it was consecutive to a mechanical trauma [6]. Literature also describes many cases of complete fracture without mechanical impact [5] more similar to our recent experience.
Particular attention must be paid to the lead failure rates in publications: The concerned Model 3501 S-ICD electrode has been distributed since June 2017 and some published results are from the previous lead version ref.3401 (not include in the recall). For example, the Praetorian randomized study [7] shows a non-inferiority of the S-ICD system vs. transvenous ICD with 849 patients enrolled from March 2011 to January 2017 (Model 3401 lead only). Part of the composite primary endpoint was lead replacement. If the occurrence of failure on the recall population remains stable around 0.3% the non-inferiority results of the Praetorian study are reassuring.
Consistent with transvenous lead data, the population at risk of S-ICD lead fracture is the young or sportive patient, especially sports with risk of trauma. For patients implanted with a Model 3501 electrode, we must pay attention to signals on both three detection vectors (artefacts, flatline on alternate vector…). The manufacturer recommendations are a weekly home monitoring follow-up and every 3 months for the face-to-face ones.
In addition to signal analysis, chest X-Ray remains the reference examination to remove doubt (both lateral and posteroanterior images must be done). Regarding the management of those failures, the extraction appears safe and easy most of the time. Behar and al. [8] reports that simple traction works for 59.4% of patients while 28.1% needs a specific sheath to avoid fibrosis issue and 9.4% needed an additional incision.
In our experience, the extraction with a sheath was easy and the decision to re-implant at 1cm more left was finally successful and a good alternative. We also keep in mind that during lead implant, an increase mechanical stress is possible due to the angle taken when pushing the lead up. To avoid this, we prefer to attach the sleeve in two times with a good thread positioning before lead insertion.
Since July 2021, an improved version of a subcutaneous lead is available. No lead fractures have been reported since the corrective action, but longer follow-up is needed to confirm these data.
Conclusion
A flat alternate vector, high impedance, and similar primary and secondary vectors are indicative of a subcutaneous lead fracture in distality of the proximal electrode. Extracting method differs if the fracture is complete or not. To preserve coil integrity, the use of a dilator sheath is highly recommended. As demonstrate here, extracting a fractured subcutaneous lead remains a safe and easy procedure.
References
- Kleemann T, Becker T, Doenges K, Vater M, Senges J, et al. Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of >10 years. Circulation. 2007; 115: 2474-80.
- Information de sécurité - Défibrillateur implantable sous-cutané. ANSM. 2021.
- Product Performance Report - Boston Scientific. 2023.
- O’Neill L, Gillis K, Wielandts JY, Hilfiker G, Knecht S, et al. Flatline on the alternate vector…Is this subcutaneous implantable cardiac defibrillator lead fractured?. Heart Rhythm Case Rep. 2021; 7: 758-761.
- Gutleben KJ, Nelovic V, Pujdak K, Werner M, Osmani I, et al. Fracture of an S-ICD lead after two prior transvenous lead-related complications with conventional defibrillators. Pacing Clin Electrophysiol. 2020; 43: 1491-1494.
- Huynh H, Siroky G, Bisht D, Lam P, Mohammad A, et al. Partial fracture of a subcutaneous ICD lead from mechanical trauma. J Clin Cardiol Cardiovasc Interv. 2019; 3: 1-22.
- Knops RE, Nordkamp LRA, Delnoy PPHM, Boersma LVA, Kuschyk J, et al. Subcutaneous or Transvenous Defibrillator Therapy. Engl J Med. 2020; 383: 526-36.
- Behar N, Galand V, Martins RP, Jacon P, Badenco N, et al. Subcutaneous Implantable Cardioverter-Defibrillator Lead Extraction: First Multicenter French Experience. JACC Clin Electrophysiol. 2020; 6: 863-870.