
Special Article: Thrombin Inhibitors
J Blood Disord. 2023; 10(1): 1074.
New Methodological Approach for Coagulation Assays Using Chicken Plasma Intrinsic Properties
Prezoto BC¹*; Falla MVA²; Lebrun I²
1Laboratory of Pharmacology, Butantan Institute, São Paulo, SP, Brazil
2Laboratory of Biochemistry, Butantan Institute, São Paulo, SP, Brazil
*Corresponding author: Prezoto BC Laboratory of Pharmacology, Butantan Institute, Av Vital Brasil, 1500, CEP 05503-900, São Paulo, SP, Brazil. Tel: +55-11-2627-9753 Email: benedito.prezoto@butantan.gov.br
Received: May 12, 2023 Accepted: June 13, 2023 Published: June 20, 2023
Abstract
Background: Sensitivity of classical coagulation assays by using mammalian plasmas to pro- and anticoagulant compounds including venom or toxins occurs on a microscale level (micrograms). Although it improves responses to agonists, recalcification triggers a relatively fast thrombin formation process. The Recalcification Time (RT) of factor XII- deficient Chicken Plasma (CP) is comparatively long (=1800 seconds) when compared to human plasma or others. Our objective was to compare its sensitivity with that presented by human plasma samples to Unfractionated Heparin (UH), a prototype anticoagulant compound, under similar conditions through rotational thromboelastometry.
Methods: To find doses of UH sufficient enough to prolong the Clotting Time (CT) parameter of these activated plasmas to values within their normal RT ranges.
Results: In total, 0.0065±0.0009 IU of UH (n=6) was detected in 260μL of CP samples, but only 0.125±0.012 IU of UH was sufficient to induce a similar effect in activated human plasma samples.
Conclusion: The higher sensitivity of CP to anticoagulants could be useful for (a) detection of anticoagulant compounds in substances of unknown origin; (b) purification procedures of anticoagulant toxins from crude animal venoms and (c) determination of relative potencies of agonists and their selective antagonists such as pharmaceutical agents, antivenoms or natural inhibitors of venom toxins with a better result in kinetic clothing parameters.
Keywords: Coagulation process; Chicken; Factor XII deficiency; Animal models; Biomedical research; Rotational thromboelastometry
Introduction
The coagulation cascade consists of a complex network of interactions resulting in thrombin-mediated cleavage of fibrinogen to fibrin, which is one major component of a thrombus. The coagulation cascade can be initiated either by the “extrinsic pathway”, the release of tissue factor leading to activation of factor VII to the tissue factor/factor VIIa complex, or by the “intrinsic pathway”, so-called contact activation leading via factors XII, XI and IX to the assembly of the tenase complex consisting of activated factors VIII and IX and Ca2+. Both complexes can activate factor X, which induces the formation of the prothrombinase complex consisting of activated factors X, V and Ca2+ on a phospholipid surface. The latter leads to the activation of thrombin, which in turn cleaves fibrinogen to fibrin [1].
Circulating blood cells, coagulation factors and vascular wall components of mammalian species are considered as targets of a variety of coagulant agonists, including venom toxins [2], which have been investigated both as laboratory reagents and as potential therapeutic agents. The study of venom pro- or anticoagulant toxins has been traditionally assessed directly by simple clotting studies on mammalian plasma samples. However, these classical coagulation assays present some limitations. For example, most of in vitro techniques designed for assaying the coagulant activity of snake venoms or toxins on mammalian plasma samples offer a single parameter for one complex enzymatic process [3]. Attempts to overcome this kind of limitation have been enacted with the use of different strategies, such as the monitoring of viscoelastic changes in plasma or Whole Blood (WB) samples with thromboelastography and, more recently, rotational thromboelastometry. These techniques improve the evaluation of the clotting process, since it monitors several parameters, such as the stages of clot initiation, formation, stability, strength and dissolution [4]. This technology has been used in various studies on the pro- or anticoagulant activities of several snake venoms or toxins on citrated human whole blood, as well as plasma samples of rats and dogs [5–10].
Citrated plasma is the substrate for almost all coagulation-specific laboratory tests and is derived from whole blood drawn into a tube containing liquid 3.2% sodium citrate at a ratio of nine parts whole blood and one part citrate. Citrate acts as a Ca2+ chelating agent to prevent coagulation of the sample so that all the clotting factors are preserved and can be evaluated. Chelation of Ca2+ determines another obvious restriction of classical coagulation assays, since non-recalcified plasma samples such as those used in the minimum coagulant dose require relatively large amounts of venom or toxins [11]. Attempt to improve sensitivity to agonists could be achieved by the addition of Ca2+ and phospholipids, since these cofactors are essential in some enzymatic steps of the coagulation cascade [12,13]. However, if higher sensitivity could be achieved, this strategy simultaneously starts and accelerates enzymatic reactions of the coagulation process; as a consequence, the time interval in which agonists can be assayed becomes limited (to around 600 seconds). This limitation associated with recalcified mammalian plasma samples becomes evident when small amounts of the proteins being tested are available; for example, during screening strategies for purification procedures of individual toxins from crude venoms or during assays for detecting the presence of pro- or anticoagulant substances in samples of unknown origins. This restricted time interval does not allow the elaboration of one typical dose–response curve to agonists or antagonists.
Another strategy to improve sensitivity to agonists by using recalcification could be achieved through the use of reptiles and avian blood or plasma samples, which present a prolonged Recalcification Time (RT) [14].
By testing recalcified factor XII-deficient Chicken Plasma (CP) samples through rotational thromboelastometry, we recently published one study in which we described that addition of the cofactors Ca2+ and phospholipids to this plasma elicited a time lapse sufficient (>1800 seconds) for the elaboration of one typical dose–response curve after testing with the in vitro procoagulant venom of the snake Bothrops jararaca (B. jararaca), displacing its sensitivity to a nanoscale range [15]. A similar strategy was later used to test the response of CP samples to the anticoagulant activity of two venom toxins: crotoxin (from Crotalus durrissus terricus snake venom) [16] and Phospholipase A2 (PLA2) from Apis mellifera bee venom [17]. However, the relative magnitude of the sensitivity of this pharmacological preparation to anticoagulant substances is generally unknown.
Classical coagulation assays such as the Prothrombin Time (PT), Activated Partial Thromboplastin Time (APTT) and Thrombin Time (TT) are global coagulation screening tests routinely used for assessment of the coagulation status in patients with suspected acquired deficiencies of coagulation factors of the intrinsic and common pathways of the coagulation system, and are also extensively used to monitor anticoagulant agents, including direct thrombin inhibitors [18,19].
In this study, we challenged chicken and human plasma samples under similar conditions through rotational thromboelastometry to compare their sensitivity to the anticoagulant effect of Unfractionated Heparin (UH), a sulfated polysaccharide and an essential drug that has been used as a clinical anticoagulant worldwide for over 90 years [20]. These assays were conducted under controlled conditions, i.e., these plasma samples were simultaneously activated with standardized doses of one classical activator of coagulation (the aPTT clot reagent, containing ellagic acid and synthetic phospholipids), taking their Recalcification Time (RT) as a reference.
The highest sensitivity of recalcified CP to the in vitro heparin anticoagulant activity (almost 19-fold higher than that presented by human plasma) described in this paper reinforces our previous findings using anticoagulant compounds such as crotoxin from Crotalus durrissus terricus venom and PLA2 from Apis mellifera bee venom.
Materials and Methods
Reagents
Activated Partial Thromboplastin Time Reagent (aPTT clot), containing ellagic acid plus synthetic phospholipids, was from BIOS Diagnostica (SP, Brazil); pooled 4% citrated normal human plasma (maintained at - 80°C) from Sigma-Aldrich, Inc. (USA); unfractioned sodium heparin (Hepamax-S) (5,000IU/mL) from swine intestinal mucosa, from Blau Farmacêutica (Cotia, SP, Brazil). All chemicals were of analytical reagent grade.
Animals
Adult female Gallus gallus domesticus chickens (1.0 to 1.7kg) were used. All birds were a donation from commercial breeding (Granja Ino, São Paulo, SP, Brazil). The animals had free access to water and food, and were kept under a 12h light/dark cycle.
The experimental protocol was approved by the Ethic Committee on Animal Use of the Butantan Institute (protocol CEUA number 6259250918).
Obtention of Citrated Chicken Plasma Samples
Blood samples were collected into syringes containing 1:10 (v/v) 3.2% trisodium citrate and then closed with cotton-yarn. Chicken plasma was obtained after centrifugation at 3000xg for 20 min at 4°C and stored at - 80°C.
Thromboelastometric Assays with Chicken or Human Plasma Samples
Standardization of the activator aPTT clot mean coagulant dose: Clotting Time (CT) parameter (in seconds, from start of the reaction to initial clot formation) of the INTEM (intrinsic pathway thromboelastometry) assay was measured using a computerized ROTEM four channel system (Pentapharm, Munich, Germany), during 3h at 37°C. In summary, before recalcification (with 20μL of 0.2M CaCl2), 260μL of human or chicken plasmas were incubated for 1 min with 60μL of 0.9% NaCl solution containing 0.9% NaCl solution [control group, so-called Recalcification Time (RT)] (Group 1) or crescent doses of the activator aPTT clot reagent, for determination of its Mean Coagulant Dose (MCD) (Group 2). The RT of CP samples in control group is relatively prolonged (=1800 seconds), when compared to that presented by human plasma (almost 600 seconds). For the elaboration of a dose–response curve of CP samples to tested doses of the activator aPTT clot reagent, reference values of 90, 900 and 1800 seconds were standardized as maximum (90%), mean (50%) and minimum (10%) coagulant responses, respectively. On the other hand, for the elaboration of a dose– response curve of human plasma samples to tested doses of the activator aPTT clot reagent, reference values of 30, 300 and 600 seconds were standardized as maximum (90%), mean (50%) and minimum (10%) coagulant responses, respectively (Figure 1).
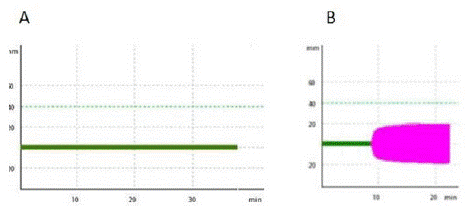
Figure 1: Recalcification time of chicken (A) or human (B) plasma samples (control groups).
Clotting Time (CT) parameter (in seconds, from start of the reaction to initial clot formation) of the INTEM (intrinsic pathway thromboelastometry) assay was measured using a computerized ROTEM four channel system, during 3h at 37 0C. Before recalcification (with 20μL of 0.2M CaCl2), 260μL of chicken (A) or human (B) plasmas were incubated for 1 min with 60μL of 0.9% NaCl solution containing 0.9% NaCl solution [control group, so-called Recalcification Time (RT)].
Standardization of the Relative Anticoagulant Dose of Heparin on Human- or Chicken-Activated Plasma Samples
Before recalcification, 260μL of chicken or human plasmas were incubated for 1 min with 50μL of 0.9% NaCl solution containing the MCD dose of the activator aPTT clot reagent plus 10μL containing 0.9% NaCl solution (Group 3), or decreasing doses of UH, to determine Relative Anticoagulant Doses (RAD) (Group 4). Concerning the CP, the RAD of UH was defined as its minimum doses (in IU) sufficient to displace CT values induced by the MCD of the activator aPTT clot reagent (900 seconds, or 50% activation) to values inside the normal range of RT (=1800 seconds in 100% of assays, considered as 100% of inhibition of activation). On the other hand, the RAD of UH upon human plasma was defined as minimum doses (in IU) sufficient to displace CT values induced by the MCD of the activator aPTT clot reagent (300 seconds, or 50% activation) to values inside the normal range of RT in 100% of assays (and considered as 100% of inhibition of activation) (Figure 2).
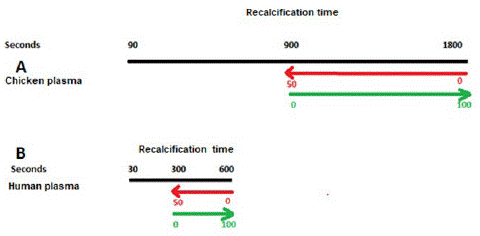
Figure 2: Protocol for determination of the activator aPTT clot mean coagulant dose and the relative anticoagulant dose of heparin on human- or chicken-activated plasma samples.
In black: Approximate recalcification time of chicken (A) and human (B) plasma samples; in red: crescent doses of the activator aPTT clot reagent decreases the clotting time parameter; in green: crescent doses of unfractionated heparin (UH) increases the clotting time parameter to values inside the normal range of recalcification time.
Statistical Analysis
Values of the CT parameter were monitored in seconds and expressed as mean ± SD of six independent experiments. One-way analyses of variance (ANOVA) comparisons between conditions were used, followed by Newman–Keuls post hoc analysis. Values defining the MCD of the activator aPTT clot reagent were determined by means of linear regression analysis, with GraphPad Prism 5.0 software (San Diego, CA, USA). p<0.05 was considered statistically significant.
Results
Standardization of the Activator aPTT Clot mean Coagulant Dose upon Chicken or Human Plasma Samples
Careful collection and centrifugation of chicken blood samples are required, since foam or bubble formation into syringes leads to cell lysis and possible activation of the coagulation process, shortening RT of control-treated samples. CT values of control- treated samples of recalcified CP (or RT) were significantly prolonged (1961±311 seconds, n = 6) when compared with those presented by human plasma (574±89 seconds, n=6). The MCD of aPTT clot reagent was the amount (in μL) that shortened the CT parameter of the control group to values situated inside a range that induced mean coagulant responses (900 and 300 seconds, for CP and human plasma samples, respectively), and considered as 50% activation. These values of the MCDs of aPTT clot reagent that shortened CT parameters of CP and human plasma to 900 and 300 seconds, respectively, were calculated by using linear regression analysis for each experimental group (Figure 2).
Standardization of the Relative Anticoagulant Dose of the UH on Chicken- or Human-Recalcified and -Activated Plasma Samples: UH presented a dose-dependent anticoagulant effect when added to CP simultaneously recalcified and activated with the MCD of the activator aPTT clot reagent, and minimum doses of 0.0065 IU±0.0009 (n=6) IU were sufficient to induce 97,5% inhibition of activation. A similar anticoagulant effect was observed upon human plasma samples, but minimum doses sufficient to induce 95% inhibition of activation were 0.125±0.012 IU (n=6) (Figure 2).
Discussion
In previous studies with crotoxin and bee venom PLA2, we found that recalcified CP samples presented sensitivity that was unusually superior to that presented by human plasma under similar conditions, when assayed for detecting the in vitro anticoagulant activities of two animal venom toxins [16,17]. Thus, our goal was to extend these studies by comparing the sensitivity of these two plasma samples in detecting the anticoagulant effect of heparin, a classical compound that has been used extensively for the last 70 years to treat thromboembolic disorders. Heparin is a highly sulfated polysaccharide that is used as a major clinical anticoagulant, and its major biological role is the regulation of the coagulation system. Besides presenting a wide range of biological roles related to inflammation, angiogenesis, growth factors, developmental process and various disease processes, heparin binds to and enhances the activity of antithrombin, a serine protease inhibitor, and targets coagulation proteins including activated factors X and II (thrombin) [21-26]. Antithrombin is present in the blood plasma of each of the terrestrial vertebrate groups, including mammals, birds, reptiles and amphibians [27].
The APTT is widely used for monitoring anticoagulation therapy with low levels of heparin (from 0.1IU/mL to approximately 1IU/mL). A therapeutic APTT range of 1.5 to 2.5 times control is widely accepted. It has been shown that this range corresponds to 0.2 to 0.4IU/mL of UH by protamine titration and 0.3 to 0.7IU/mL by anti-Xa assay [18-19].
In this study, by using the rotational thromboelastometry technique, our strategy was: (i) first, to define normal range values of the CT parameter of the INTEM profile (recalcification time) of recalcified chicken and human plasma samples. Values of the CT parameter found (574±89 and 1961±311, for human and chicken plasmas, respectively) presented themselves according to previous studies [15-17]; (ii) second, to standardize the MCD of the activator aPTT clot reagent, since values of the CT parameter situated between the minimum and maximum procoagulant response of these plasmas may be considered as a region over which responses are most sensitive; (iii) finally, to quantify the anticoagulant effect of UH, defining its minimum doses (in IU) sufficient to displace CT values induced by the MCD of the activator aPTT clot reagent to values inside the normal ranges of the RT of these plasma samples, in 100% of assays.
We reported earlier that CP samples presented sensitivity to the anticoagulant effect of bee venom PLA2 almost 20-fold higher than that presented by human plasma samples. Accordingly, a sensitivity almost 19-fold higher to the in vitro anticoagulant effect of UH was found here, since the RAD was 0.125±0.012 IU and 0.0065IU±0.0009 IU for human and CP samples, respectively.
Below, we present some considerations justifying why, in our opinion, CP samples present a sensitivity two orders of magnitude higher than that presented by human plasma samples to the anticoagulant effect of UH.
Time to spontaneous clot formation after recalcification is highly variable in the plasma of mammal and non-mammal species. It has been suggested that slow clotting of reptilian and avian blood, and the complex hemostatic mechanism with particularly high platelet counts and rapid clotting of the blood of carnivores, is a rather extreme example of the way in which evolutionary trends in physiology and lifestyle may place differing demands on the coagulation system [28].
Four constituents form the mammalian intrinsic pathway of coagulation: the trypsin-type serine proteases factors XIIa, XIa, plasma kallikrein and the cofactor high molecular weight kininogen [29]. Presence of factor XII (Hageman factor) is one key variable that negatively influences the RT of plasma samples [30]. Accordingly, extreme slowness in spontaneous in vitro thrombin/fibrin generation, even after the addition of Ca2+ ions, is a hallmark of the plasma of patients with a factor XII deficiency [31].
The blood coagulation system has been studied in several avian species and the findings are particularly interesting because they suggest an almost total absence of surface contact activation of an intrinsic pathway [32]. In fact, the main difference between the human and chicken coagulation process is that the latter presents functional factor XII deficiency, since the factor XII gene is completely missing in the chicken genome [33]. However, chicken plasma does possess a fully functional extrinsic pathway, and its key coagulant proteins (factors V and X) could be considered as suitable targets or substrates for coagulant toxins from several animal venoms [34,35]. Moreover, in contrast with the hemostatic system of some reptilian species [36-40], avian species do not present significant amounts of natural inhibitors against snake venom proteins.
Conclusion
In conclusion, we propose this functional assay as a sensitive and alternative tool for screening anticoagulant toxins during purification procedures of crude animal venoms or for the detection of anticoagulant compounds in substances of unknown origins. The relatively large RT presented by CP elicits an elaboration of one dose–response curve for searching statistically different doses inducing minimal, mean or maximum coagulant responses to procoagulant agonists such as the activator aPTT clot reagent or some snake venoms, which in turn determines the relative potencies of their selective antagonists such as prototypes of pharmaceutical agents, specific antivenoms [15] or natural inhibitors of venom toxins.
Author Statements
Acknowledgments
The authors would like to acknowledge (i) the staff within the Laboratory of Pharmacology, from the Butantan Institute, (ii) Granja Ino, São Paulo, SP, Brazil for providing the chickens,
Author Contributions
Conceptualization, Benedito C. Prezoto; methodology, Benedito C. Prezoto, Ivo Lebrun and Monica V. A. Falla; software, Benedito C. Prezoto; validation, Benedito C. Prezoto, Ivo Lebrun and Monica V. A. Falla; formal analysis, Benedito C. Prezoto, Ivo Lebrun and Monica V. A. Falla; investigation, Benedito C. Prezoto, Ivo Lebrun and Monica V. A. Falla; resources, Benedito C. Prezoto and Ivo Lebrun; data curation, Benedito C. Prezoto and Ivo Lebrun; writing—original draft preparation, Benedito C. Prezoto and Ivo Lebrun; writing—review and editing, Benedito C. Prezoto and Ivo Lebrun; visualization, Benedito C. Prezoto, Ivo Lebrun and Monica V. A. Falla; supervision, Benedito C. Prezoto and Ivo Lebrun; project administration, Benedito C. Prezoto and Ivo Lebrun; funding acquisition, Benedito C. Prezoto and Ivo Lebrun. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), grant number 2009/54620-4.
Conflicts of Interest
The authors declare no conflict of interest. The funding body for this study had no role in the design of the study; in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Institutional Review Board Statement
The experimental protocol was approved by the Ethic Committee on Animal Use of the Butantan Institute (protocol CEUA number 6259250918).
References
- Smith SA, Travers RJ, Morrissey JH. How it all starts: Initiation of the clotting cascade. Crit Rev Biochem Mol Biol. 2015; 50: 326-336.
- Du X-Y, Sim DS, Lee W-H, Zhang Y. Blood cells as targets of snake toxins. Blood Cells Mol Dis. 2006; 36: 414-421.
- Isbister GK. Procoagulant snake toxins: laboratory studies, diagnosis, and understanding snakebite coagulopathy. Semin Thromb Hemost. 2009; 35: 93- 103.
- Whiting D, DiNardo JA. TEG and ROTEM: Technology and clinical applications. Am J Hematol. 2014; 89: 228–232.
- Dambisya YM, Lee TL, Gopalakrishnakone P. Action of Calloselasma rhodostoma (Malayan pit viper) venom on human blood coagulation and fibrinolysis using computerized thromboelastography (CTEG). Toxicon. 1994; 32: 1619–1626.
- Dambisya YM, Lee TL, Gopalakrishnakone P. Anticoagulant effects of Pseudechis australis (Australian king brown snake) venom on human blood: A computerized thromboelastography study. Toxicon. 1995; 33: 1378–1382.
- Nagel SS, Schoeman JP, Thompson PN, Wiinberg B, Goddard A. Hemostatic analysis of dogs naturally envenomed by the African puffadder (Bitis arietans) and snouted cobra (Naja annulifera). J Vet Emerg Crit Care. 2014; 24: 662–671.
- Hiremath V, Nanjaraj Urs AN, Joshi V, Suvilesh KN, Savitha MN, et al. Differential action of medically important Indian BIG FOUR snake venoms on rodent blood coagulation. Toxicon. 2016; 110: 19–26.
- Nielsen VG, Boyer LV, Redford DT, Ford P. Thrombelastographic characterization of the thrombin-like activity of Crotalus simus and Bothrops asper venoms. Blood Coagul Fibrinolysis. 2017; 28: 211–217.
- Nielsen VG, Sanchez EE, Redford DT. Characterization of the rabbit as an in vitro and in vivo model to assess the effects of fibrinogenolytic activity of snake venom on coagulation. Basic Clin Pharmacol Toxicol. 2018; 122: 157–164.
- Theakston RDG, Reid HA. Development of simple assay standard procedures for the characterization of snake venoms. Bull World Health Organ. 1983; 61: 949- 956.
- O’Leary MA, Isbister GK. A turbidimetric assay for the measurement of clotting times of procoagulant venoms in plasma. J Pharmacol Toxicol Methods. 2010; 61: 27–31.
- Mann KG, Jenny RJ, Krishnaswamy S. Cofactor proteins in the assembly and expression of blood clotting enzyme complexes. Annu Rev Biochem. 1988; 57: 915–956.
- Spurling NW. Comparative physiology of blood clotting. Comp Biochem Physiol A Physiol. 1981; 68: 541-548.
- Oguiura N, Kapronezai J, Ribeiro T, Rocha MM, Medeiros CR, et al. An alternative micromethod to access the procoagulant activity of Bothrops jararaca venom and the efficacy of antivenom. Toxicon. 2014; 90: 148–154.
- Prezoto BC, Tanaka-Azevedo AM, Marcelino JR, Tashima AK, Nishiduka ES, Kapronezai J, et al. A functional and thromboelastometric-based micromethod for assessing crotoxin anticoagulant activity and antiserum relative potency against Crotalus durissus terrificus venom. Toxicon. 2018; 148: 26–32.
- Prezoto BC, Oguiura N. Factor XII-Deficient Chicken Plasma as a Useful Target for Screening of Pro- and Anticoagulant Animal Venom Toxins. Toxins (Basel). 2020; 12: 79.
- Palmer RL. Laboratory diagnosis of bleeding disorders. Basic screening tests. Postgrad Med. 1984; 76: 137-148.
- Levy JH, Szlam F, Wolberg AS, Winkler A. Clinical Use of the Activated Partial Thromboplastin Time and Prothrombin Time for Screening: A Review of the Literature and Current Guidelines for Testing. Clin Lab Med. 2014; 34: 453-477.
- Onishi A, Ange KS, Dordick JS, Linhardt RJ. Heparin and anticoagulation. Front Biosci. 2016; 21: 1372-1392.
- Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, et al.. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012; 141: e326S- e350S.
- Garcia DA, Baglin TP, Weitz, JI, Samama MM. Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012; 141: e24S-e43S.
- Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012; 141: e419S- e496S.
- Linhardt RJ. Heparin: structure and activity. J Med Chem. 2003; 46: 2551- 2554.
- Hirsh J, Warkentin TE, Raschke R, Granger C, Ohman EM, et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety. Chest. 1998; 114: 489S- 510S.
- Hirsh J, Anand SS, Halperin JL, Fuster V, American Heart Association. Guide to anticoagulant therapy: Heparin: a statement for healthcare professionals from the American Heart Association. Circulation. 2001; 103: 2994-3018.
- Jordan RE. Antithrombin in vertebrate species: conservation of the heparin- dependent anticoagulant mechanism. Arch Biochem Biophys. 1983; 227: 587- 595.
- Spurling NW. Comparative physiology of blood clotting. Comp Biochem Physiol. 1981; 68A: 541-548. Weidmann H, Heikaus L, Long AT, Naudin C, Schlüter H, Renné T. The plasma contact system, a protease cascade at the nexus of inflammation, coagulation and immunity. Biochim Biophys Acta Mol Cell Res. 2017; 1864: 2118-2127.
- Weidmann H, Heikaus L, Long AT, Naudin C, Schlüter H, Renné T. The plasma contact system, a protease cascade at the nexus of inflammation, coagulation and 10 immunity. Biochim Biophys Acta Mol Cell Res. 2017; 1864: 2118-2127.
- Moiz B, Sadiq MW, Javed MA, Baqir Jafry BH, Anees M. Prolonged activated partial thromboplastin time secondary to factor XII deficiency in two surgical patients. Oxf Med Case Reports. 2021; 2021: omaa146.
- Ratnoff OD, Colopy JE. A familial hemorrhagic trait associated with a deficiency of a clot-promoting fraction of plasma. J Clin Invest. 1955; 34: 602–613.
- Archer RK. Physiology and Biochemistry of the Domestic Fowl. (Edited by BELL D J and FREEMANB M, editors. In: Blood Coagulation. Academic Press. 1971; 897-891.
- Ponczek MB, Gailani D, Doolittle RF. Evolution of the contact phase of vertebrate blood coagulation. J Thromb Haemost. 2008; 6: 1876–1883.
- Johnson GS, Turrentine MA, Swayne DE. Coagulation of plasma from the chicken (Gallus domesticus): phospholipids influence clotting rates induced by components from Russell’s viper venom. Comp Biochem Physiol B. 1985; 82: 647-653.
- Bernardoni JL, Sousa LF, Wermelinger LS, Lopes AS, Prezoto BC, et al. Functional variability of snake venom metalloproteinases: adaptive advantages in targeting different prey and implications for human envenomation. PLoS ONE. 2014; 9: e109651.
- Nahas L, Kamiguti AS, Sousa e Silva MC, Ribeiro de Barros MA, Morena P. The inactivating effect of Bothrops jararaca and Waglerophis merremii snake plasma on the coagulant activity of various snake venoms. Toxicon. 1983; 21: 239-246.
- Fortes-Dias CL, Fonseca BC, Kochva E, Diniz CR. Purification and properties of an antivenom factor from the plasma of the South American rattlesnake (Crotalus durissus terrificus). Toxicon. 1991; 29: 997-1008.
- de Oliveira EP, Tanizaki MM. Effect of a proteinase inhibitor from the plasma of Bothrops jararaca on coagulant and myotoxic activities of Bothrops venoms. Toxicon. 1992; 30: 123-128.
- Thurn MJ, Broady K, Mirtschin PJ. Neutralisation of tiger snake (Notechis scutatus) venom by serum from other Australian elapids. Toxicon. 1993; 31: 909-912.
- Smith A, Marshall LR, Mirtschin PJ, Jelinek GA. Neutralisation of the clotting activity of Australian snake venoms by snake plasma. Toxicon. 2000; 38: 1855-1858.