
Research Article
Austin J Anal Pharm Chem 2024; 11(1): 1169.
Increased Intracellular Insulin from Differentiated Stem Cells to Insulin- Producing Cells Incorporating B-Carotene
Francisco Josué Avelar Rodríguez¹; Sanghamitra Khandual²; Jorge Gaona Bernal³; Erika Nahomy Marino Marmolejo¹; Jorge Bravo-Madrigal¹; Flor Yohana Flores Hernández¹*
1Department of Medical and Pharmaceutical Biotechnology of Center for Investigation and Assistance in Technology, México
2Department of Industrial Biotechnology of Center for Investigation and Assistance in Technology, México
3Department of Microbiology and Pathology, Centro Universitario de Ciencias de la Salud, Universidad de Guadalajara México
*Corresponding author: Flor Yohana Flores Hernández Center for Investigation and Assistance in Technology, Design for State of Jalisco, C.P. 45019, Zapopan, Jalisco, Mexico. Tel: +52 3333455200, Ext. 1328; Fax: +52 3333455200 1001 Email: fflores@ciatej.mx
Received: March 04, 2024 Accepted: April 10, 2024 Published: April 17, 2024
Abstract
Analyzes the effect of β-Carotene (BC) in the processes of differentiation of adipose tissue Human Mesenchymal Stem Cells (hADMSCs) and dental pulp Human Mesenchymal Stem Cells (hDPMSCs) for obtaining Insulin-Producing Cells (IPCs). The BC present in the methanolic extract was analyzed and quantified by UV-VIS, TLC and HPLC methods.
The cell viability test was done by flow cytometry, a concentration of 1.5 μM of BC or AP extract no cytotoxic effect on stem cells, so this concentration was used for the Human Mesenchymal Stem Cells (hMSCs) differentiation protocols for obtaining IPCs. The differentiation tests were done with the AP extract or β-carotene standard addition which demonstrates the increased cell differentiation percentages by the presence of intracellular insulin. This was more outstanding in the hMSCs that were stimulated with the AP in stage 3 and applied at all stages mainly in hDPMSCs, reaching up to 74% of the insulin-positive population respect to 11% where the molecules were not added. RT-PCR was performed for the level of gene expression like MAFA, PDX1 and NKX6.1 and those are found well expressed on differentiated cells.
This could be relevant in cell therapy in diabetes, where inclusion of AP extracts or BC may improve yield of IPCs.
Keywords: Arthrospira platensis; β-carotene; Differentiation; Insulin-producing cells; Mesenchymal stem cells; Microalgae
Introduction
Diabetes Mellitus (DM) is a chronic metabolic disorder with rapid increase in prevalence, affecting 382 million people worldwide, becoming one of the leading diseases of the 21stcentury [1], DM is the result of inadequate supply of functional β-cells, as these cells are responsible for the production of insulin. Diabetes therapy mainly involves insulin, drugs that enhance insulin secretion or inhibitors of the signaling of endogenous glucose production [2]. However, it is important what kind of cell therapy in diabetes considers the replacement of β-cells derived from human stem cells by the induction of endogenous regeneration through the formation of new cells expressing insulin either by conversion of a differentiated cell type (trans-differentiation) or differentiation of progenitors (neogenesis) in order to cure the disease [3].
Specifically, the use of Human Mesenchymal Stem Cells (hMSCs) has a high potential use in various therapies, due to their multipotent self-renewal capacity as well as potential to differentiate into mesodermal, endodermal and ectodermal lineages. The hMSCs demonstrate expression of specific cell surface molecules such as CD105, CD73 or CD90 and CD44. The use of hMSCs presents several advantages such as the immunomodulatory potential, the reduction of problems related to tumorigenicity and that are found in adult tissues, so that their use may overcome potential ethical issues. Several tissues that contain stem cells and these areas they have been called "niches", such as Human Adipose Tissue (hADMSCs), Human Bone Marrow (hBMMSCs), Human Wharton's Gelatin (hWJMSC), Human Umbilical Cord Blood (hUCBMSC) and Human Dental Pulp (hDPMSCs) [1]. hMSCs can be differentiated into various types of specialized cells such as Insulin-Producing Cells (IPCs). Several protocols for differentiation have been implemented to obtain IPCs, which vary according to the type of mesenchymal stem cell. In general, the protocols are carried out in three stages, where hMSCs are directed towards endoderm, later to endocrine progenitors and finally towards IPCs [4]. Due to the low efficiency of differentiation for obtaining the cells and efficiency of the functionality of these cells, there is a great interest the development of an improved process of in vitro differentiation, but information regarding the factors of the efficient differentiation of hMSCs to β-cells are yet unknown [1]. Specifically, hADMSCs and hDPMSCs are important cell sources and frequently used in the field of tissue engineering and/or regenerative medicine. Due to its easy disponibility, pluripotency capacity and easy proliferation qualities, these cells can be exploited in cell therapy for diabetes to increase the yield of IPCs.
One of the strategies that is followed to increase the efficiency of differentiation protocols of differentiation is the search for new molecules, including those from phycoextracts. Several works focused on the study of the therapeutic activity of BC analyze the inhibition of tumorigenesis in stem cells and discuss the activity of this molecule in the differentiation of these cells by regulating differentiation markers, such as Drosophila Delta-Like 1 Homologue (DLK1) which is a member of the Epidermis Growth Factor (EGF) similar to the family of homeotic proteins and is known to modulate the differentiation signaling in adipocytes and several types of stem cells. However, little is known about the role of this type of extracts and molecules in a process of differentiation of hMSCs towards IPCs cells [5].
Arthrospira Platensis (AP) is a blue-green algae belonging to the family of cyanobacteria, rich in bioactive compounds, such as proteins, lipids, carbohydrates, trace elements (zinc, magnesium, manganese, selenium), riboflavin, tocopherol, and a-linoleic acid, has a 62% amino acid content and is rich in vitamin B12 and it contains a whole spectrum of natural mixed phytopigments. Among them, carotenoids and phycocyanins, some of these compounds have antioxidant activity such as β-Carotene (BC) [6]. In spirulina, BC represents 67–79%, and it has been reported that these protections from photo oxidative damage and BC holds the prime position in provitamin A activity. BC is a known antioxidant and precursor of the retinoic acid molecule of relevance in differentiation processes [5].
AP and its extracts are widely used as nutrients for humans and animals, natural colorants in food and cosmetics and nutraceuticals and food additives for products of the pharmaceutical industry, BC protect against certain chronic diseases such as cancer, diabetes, cardiovascular diseases etc, [7]. This microalgae extracts have been attributed antidiabetic properties due to the induction of recovery of damaged pancreatic β-cells through its properties as antioxidants, anti-inflammatory and anti-apoptotic [8]. But there are no reports in case of stem cell differentiation to produce IPCs with phycoextract inclusion.
In this work, we with AP extract and BC inclusion in each three stages and throughout the differentiation process [2] and focused on the evaluation during the differentiation process of hADMSCs and hDPMSCs to IPCs.
Material and Methods
Extraction Process
Arthrospira Platensis LB-23 (from university of Texas, Austin) was cultivated in UTEX spirulina medium, the biomass obtained was crushed in a mortar with glass beads and adding methanol (10 ml/g) at 25°C and then centrifuged at 4°C at 10,000 rpm for 15 minutes. The supernatant was evaporated at 30°C in a Speedvac (Vacufuge-Eppendorf), then, the extract was solubilized in Phosphate-Buffered Saline (PBS) (1:10 v/v) and filtered with Whatman filters of 0.22 μg PES (Polyethersulfon, 28420282). The efficiency of extraction was calculated according to the following formula:
Analysis of AP Extract by TLC, UV/VIS AND HPLC
For qualitative analysis of BC, Thin Layer Chromatography (TLC) technique was used. Silica gel plates were used as a stationary phase, mounted on 20 x 10 cm glass and a mixture of Hexane: Acetone used at a proportion of 70:30 (v/v), the extract was analyzed together with the standard BC (C9750-5G Sigma-Aldrich), with the running time of 20 minutes [9]. The Rf was calculated with the following formula:
A spectrophotometric method was also used for quantification at a wavelength of 455 nm [10], by reading in triplicate and comparing with a standard curve constructed using commercially available BC (C9750-5G-SIGMA) in a plate reader (Eon BioTek). Finally, an HPLC analysis was performed for quantitative analysis. The standard of BC was diluted in acetone for standard curve generation and sample quantification, using a high-efficiency liquid chromatograph (Varian ProStar) with autosampler, a Luna column was used (LC column 3μm-Phenomex 00F-4162-E0), as a mobile phase a mixture of Hexane: Acetone 82:18 (v / v) was used. Additionally, a standard of astaxanthin was included using the same conditions.
Characterization of hMSCs
The hADMSCs were obtained from ATCC (PCS-500-011, lot 62098855), with hMSCs characterization certificate. The hDPMSCs were isolated from the third molar from a 22-year-old North American male patient, all procedures were carried out in accordance with the relevant institutional laws and guidelines and approved by the appropriate institutional committees. Informed consent was obtained for experimentation with human subjects. The dental piece was extracted by exodontia collected under the ethical criteria without reported pathologies. The procedures to isolate the stem cells of the dental pulp were those mentioned by Karamzadeh [11], modifying the enzymatic solution, the digestion of the dental pulp was carried out by adding 1ml of 0.025% trypsin, the obtained cells they underwent phenotypic characterization, according to International Society of Cell Therapy (ISCT).
Flow cytometry analysis was performed to identify membrane markers for hMSCs like CD44, CD73, CD90, and CD105 as well as the absence of hematopoietic markers using the analysis kit BD (562245). Multi-differentiation was also performed for the hDPMSCs to osteocytes, adipocytes, and chondrocytes following the specifications of the ISCT. For this purpose, cells were cultured for 24 days in specific differential media under standard culture conditions like for the case of osteoblasts, the Human Osteoblast Differentiation medium was used (Sigma Aldrich 417D). Later Von Kossa staining was used to observe calcium deposits, which was done by adding 500 μl of 5% silver nitrate (AgNO3) and subjected to UV radiation at 120 μJ/cm2 for 5 minutes. For the differentiation of adipocytes, the Human Adipocyte Differentiation medium (Sigma Aldrich 811D) was used. The staining with oily red was used to demonstrate the presence of fat vacuoles. After the culturing the cells with Chondrocyte Differentiation medium (Sigma Aldrich 411D), it was stained with a solution of 1% alcian blue. hDPMSCs without subjecting differentiation treatment, were subjected to the same stains, these being the controls.
Viability Assay
Both types of hMSCs were grown in D'MEM/F-12 (Sigma D8437) enriched with L-glutamine and 10% FBS (Fetal Bovine Serum) under standard culture conditions. They were placed in six-well culture plates, utilizing different concentrations of the AP extract and BC standard diluted with culture medium DMEM/F-12 free of FBS for 72 hours. The cells were exposed to the extract and to the standard at different concentrations (3, 1.5, 0.75, 0.375 and 0 μM) under standard culture conditions at 37°C, 95% relative humidity and 5% CO2.
A follow-up was made by microscopic observation to visualize if there was cellular damage in terms of morphological changes or cellular detachment produced by the AP extract an BC. Viability analysis was performed by cytometry, the cells were harvested using Trypsin/EDTA (ATCC PCS-999-003), and the cell pellet was resuspended in PBS. Then viability test was carried out by flow cytometry using the BD Cell Feasibility Kit viability kit (349383) and the BD Accuri C6 flow cytometer, using the Side Scatter (SCC) and Forward Scatter (FCC) graphics and the FL3-FL1 channel.
Differentiation of hMSCs into Insulin-Producing Cells
Differentiation of hADMSCs
The process of differentiation of the hADMSCs was carried out using the protocol by Chandra [12]. This process consisting of three stages: stage 1 (of hMSCs to meso-endodermal cell; stage 2 (of meso-endoderms to endocrine progenitors) and stage 3 (of endocrine progenitors to IPCs), during the experiment, AP extract was added to cultures in all the stages at a concentration of 1.5 μM, as well as a control of differentiation without AP extract and a control without differentiation protocol was maintained. In other experiments, AP extract was added at stage-1 only, stage-2 only and stage-3 only respectively. The same process was followed the BC standard was used.
Differentiation of hDPMSCs
The process of differentiation of the hDPMSCs was carried out by using the protocol established by our research group, which consisted of three stages. In the first stage, serum free D´MEM/F-12, 17.5 mM Glucose, Activin A 100 ng/mL and CHIR 3μM was used for five days at 37 °C and 5% CO2. On the fifth day the change was made to stage 2 which contained serum-free D´MEM/F-12, 17.5 mM glucose, FGF 4 ng/mL, IGF 50 ng/mL, Noggin 100 ng/mL, 1μM Dorsomorphin, 2 μM Retinoic Acid for five days. On the tenth day the change was made to stage 3 which contained serum-free DMEM F-12, 17.5 mM glucose, 10μM Forskolin, 3 μM Taurine, 10mM Nicotinamide, Dexamethasone 10 μM, Supplement B27 1% for five days. During the experiment, AP extract was added to culture at 1.5 μM, as well as a control of differentiation without AP extract and a control without undergoing the protocol differentiation was maintained. In other instances, AP extract was added at stage-1 only, stage-2 only and stage-3 only respectively. The same process was followed to use the standard of BC.
Analysis of Intracellular Insulin
Once the differentiation protocols were concluded, the cells were harvested with scrapper, then stained with anti-insulin antibodies Alexa Fluor 647 (565689) and analyzed in a Accuri C6 Flow Cytometer BD, permeabilizing the hDPMSCs and hADMSCs with methanol: acetone 1:1 (v/v) samples in the flow cytometer in channel FL-3 at 670 nm.
Gene Expression Study
Gene expression profiling was done to determine in vitro cellular responses of differentiation of hMSCs with and withouth β-carotene. At the end of each differentiation stage, cells were collected to carry out an RT-PCR analysis in order to look for MAFA, PDX1 and NKX6.1 genes transcripts, β-actin was used as a reference gene (Table 1).
NCBI RNA
referenceSequences
Amplicon
Tm
F
R
MafA
NM_201589
CTTCAGCAAGGAGGAGGTCA
TTGTACAGGTCCCGCTCTTT
195
59
Nkx 6.1
NM_006168
CTGGAGAAGACTTTCGAACAA
AGAGGCTTATTGTAGTCGTCG
239
57
Pdx1
NM_000209
TCCTACAGCACTCCACCTTG
ACTGGCATCAATTTCACGGG
227
59
β-actina
NM_001101.3
GGAGTCCTGTGGCATCCACGAAACTAC
CACATCTGCTGGAAGGTGGACAGCG
261
65
Table 1: Sequences of PCR primers.
RNA Harvest
For the extraction of RNA, the RNA extraction kit was used (Promega Z6012), cell concentration of 1x106. RNA quantification was performed with the Quubit® RNA Assay kit, reading in the Qubit 3 fluorometer and was stored at -85°C until further molecular analysis.
Reverse Transcript (RT)
2 μg of extracted RNA was reverse transcribed to cDNA using a commercially available kit for RT, the RT Promega GoScriptTM Reverse Transcription System kit was used (A5001), then it was taken to the thermal cycler and a cycle was run in the ProFlex PCR. The cDNA obtained was further amplified by polymerase chain reaction.
Polymerase Chain Reaction (PCR)
System thermocycler (Life technologies) with the following temperature cycles: 25°C for 5 minutes, 42°C 1 hour, 70°C 15 minutes. For PCR, the Promega kit (M7806) was used with the ProFlex PCR System thermocycler running 40 cycles at the following temperatures for different genes: 95°C (2 minutes), 95°C (30 seconds); MAFA 57°C; NKX6.1 59°C; and PDX 1 59°C (30 seconds), 72°C 5 minutes, 4°C without time limit. 2% agarose gels were made for electrophoresis (Sigma A9539) in TBE 0.5x buffer under the following conditions: 60 Volts for one hour and 15 minutes, in a BIO-RAD development chamber and a power source of the same brand. Later, gels were stained for visualization with GelRed (Biotum 41003) and images were revealed in a BIO documenter RAD GelDocTM XR. The marker used in each reaction was a 100-Base Pair (bp) ladder.
Statistical Analysis
The Kruskal-Wall is test was used. Statistical analysis was carried out in STATGRAPHICS software (Centurion XVI). A p value of (0.05) was considered significant.
Results
Obtention of Extracts
AP was obtained from recently harvested biomass with a yield of BC of 1.26% of the biomass. The content of BC was calculated in the extract after filtration with 0.2 um syringe filters. There are some reports indicating that BC content is 80% of the carotenoids present in AP extract, the remainder consisting mainly of phycoxanthin and cryptoxanthin. Each gram of dry spirulina contains total carotenoid values like 4.43±0.03 mg/g, among all trans-β-carotene, all-trans-zeaxanthin, 9-cis-β-carotene, and diatoxanthin were found to be the major carotenoids present in spirulina. The content of all-trans-β-carotene was highest among the four major carotenoids in A. platensis [13]. But in our case, we generated it from recently harvested weight biomass to use it more effectively as crude extract form for cell line application. Our value shows the difference in BC content reported is within the range and may be due to processing differences and filtration.
Analysis of AP Extract by TLC, UV/VIS and HPLC
Analysis by Thin Layer Chromatography (TLC).
The TLC picture showed that the AP extract contains BC according to the calculation of the retention factor, Rf = 0.910. For BC the Rf value of the commercially available standard was used (Figure 1A). It also contains some other pigments like chlorophyll and lutein. This confirms the presence of BC physically our AP extract.
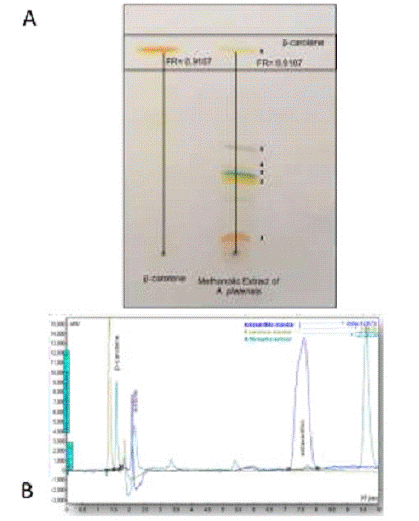
Figure 1: A) TLC plate showing the presence of carotenes in the AP extract. B) 628 Chromatogram of the AP extract. Shows the peaks corresponding to the presence of BC and 629 probable castaxanthin/zeaxanthin presence in the extract.
Previous studies showed that Arthrospira platensis extract contains the following pigments: echinenone, canthaxanthin, zeaxanthin, myxoxanthophyll, chlorophyll a, pheophytin a, β-carotene and a-carotene [14,15]. In our study we also found six bands of pigments that may represents: 1. Canthaxanthin/zeaxanthin or astaxanthin, 2. Lutein, 3. Chlorophylls, 4. Myxoxanthophyll 5. Pheophytene and 6. BC. As we have only standard sample of BC and this pigment was our point of interest to study, we confirmed the presence of the same pigment in our crude extract we used [16].
There are reports that BC induces neuronal cell differentiation, concomitant with a marked increase in the phosphorylation of extracellular signal-regulated kinases (ERK, p42/p44). Currently, little is known about the role of BC in the differentiation and self-renewal characteristics of stem cells. So here in our study we evaluated whether BC has a role or not in stem cell differentiation both as a crude phytoextract level and in its purified form.
Analysis by UV/VIS Spectrometry
The BC content of AP extract was estimated by a UV/VIS spectrometry (Eon BioTek) using a calibration curve prepared with commercially available BC. The working stock showed a concentration of 19.65 mg of β-carotene/mL of AP extract.
In the first step for determining the overall quantity of BC present in our sample, we used a spectrophotometric method. The absorbance at 455 nm of a solution containing a known amount of the standard was taken and the spectrophotometric quantity was calculated by using Beer’s Law. The accuracy of the spectrophotometric quantity and quality was compromised if the solution contains other components that also absorb at the same wavelength, therefore, the presence of interfering components was further determined by chromatographic analysis. It was clear from the TLC analysis that it also contains chloro phylls, but these pigments do not absorb at the same wavelength as BC (Figure 1).
HPLC Analysis
At the second step in our characterization of AP extract was the determination of the chromatographic purity of the crude extract. The standard was analyzed chromatographically, the chromatographic purity and presence of BC was conformed relative to all peaks in the chromatogram, because the total area of all the peaks represents the total spectrophotometric absorbance.
The HPLC analysis showed that the AP extract contains BC with retention times very close to the standard of BC that is found at 1.5 minutes, and another peack close to astaxanthin standard at minute 7.7. A calibration curve was obtained using standards of BC and astaxanthin (Figure 1B).
AP extract used in our study did not shows any other distinct peak closer to a and β-carotene peaks. The other smaller peaks in Figure 1B, were very far in the spectra as to interfere with the spectrophotometric determination we used for quantification purposes. We found six picks here, which is similar in agreement with the number of bands separated in TLC plate (Figure 1). The main two picks found and conformed was BC and a-carotene. We think that the other peak closest to astaxanthin may be castaxanthin/zeaxanthin, but this remains to be determined as we lack of all the carotenoid standards.
Characterization of hMSCs
Isolation of hDPMSCs
A culture of the pulp tissue from the dental organ, third molar tooth, was obtained, with typical fibroblast morphology with adherence to the tissue culture flask (Figure 2A) fulfilling the first criteria indicated by the International Society of Cell Therapy [17]. Once the dental piece was processed to obtain the hDPMSCs, we observed by means of inverted microscopy that adherent cells when proliferating, formed rosettes. This process took approximately 15 days until we performed a subculture to expand the cells and achieve a working stock for subsequent analyzes. In this process, it was observed by optical microscopy that the adhered cells had typical fibroblastic spindle morphology.
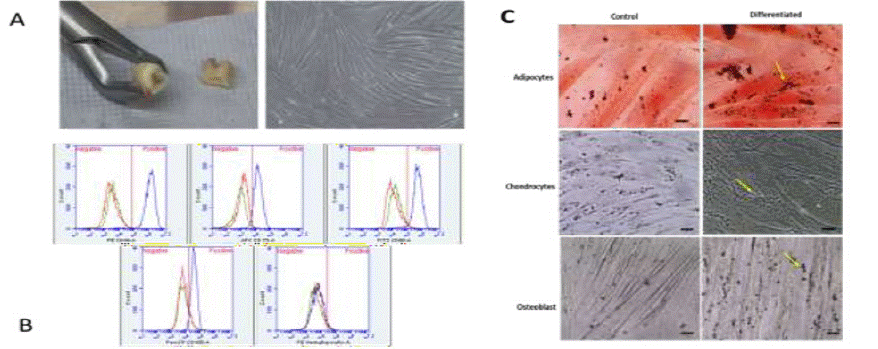
Figure 2: A. Cell culture of the pulp tissue of the dental organ. Obtaining the pulp tissue of the third molar, the fractionated third molar dental piece is shown, exposing the pulpal cavity from which the tissue was obtained and adherent cells in culture with fibroblastoid morphology, showing a high cell confluence. B. Phenotypic analysis of membrane markers flow cytometer for hDPMSCs. C. Differentiation of the hDPMSCs to cell types: adipogenic, where the conditions of the lipids inside the cells are observed; chondrocytes, where the polysaccharides are observed; osteoblastic, where calcium deposits stained in black are observed.
Phenotypic Characterization of hDPMSCs.
Phenotypic characterization of cells for mesenchymal markers showed 94.7% positivity for CD90 and CD73, 98.2% with CD44, 92.6% with CD105 and the content of hematopoietic cells was <2% (Figure 2B). Adult mesenchymal stem cells derived from human pulp dentine tissue (hDPMSCs) were obtained from a culture of the adherent cell population. Similar results found in other assays with mesenchymal cells such as [18].
These phenotypic characteristics are described by various authors where they define these membrane markers to identify hMSCs, and we demonstrate the presence of these through cell surface antigens, as well as the absence of hematopoietic markers such as CD34, CD11b CD45, CD19, and HLA- DR. This phenotypic characterization of the hMSCs is of relevance to have evidence that the studies are indeed based on analysis with this cell type [19].
Multi-Differentiation
The hDPSMCs were culture in the presence of differentiation media, then monitored by microscopy and the formation of lipid vacuoles was demonstrated by oil red staining in differentiated cells transformed to adipocytes. On the order hand, cells differentiated towards chondrocytes, after the staining with alcian blue, showed the blue lines produced by collagen fibers that are a characteristic of chondrocytes. Finally, the Von Kossa stain evidenced calcium deposits present in the cells differentiated towards osteocytes, Figure 2C.
These characteristics are carried out in several works in which hMSCs are used to demonstrate its multipotentiality, the images (Figure 2C) show that the cultured hDPMSCs have the capacity to be multidifferentiated and these strains, the osteoblastic cells, the calcium deposit extracellularly, the chondrogenic cells where the acidic polysaccharides found in the stained cartilage and the adipogenic cells where the neutral lipids are stained in the cells after the induced differentiation are observed, observing lipid droplets inside the cells stained red, these results show similarity with others jobs [20].
Viability Assay
Monitoring hMSCs Exposed to the Extract by Microscopy
In the case of hADMSCs and hDPMSCs no evident morphological changes were observed when exposed to the various concentrations of BC or AP extract compared to the control at the end of the 72 hours of exposure (Figure 3A). Hence 1.5 μM/mL concentration was selected for subsequent tests.
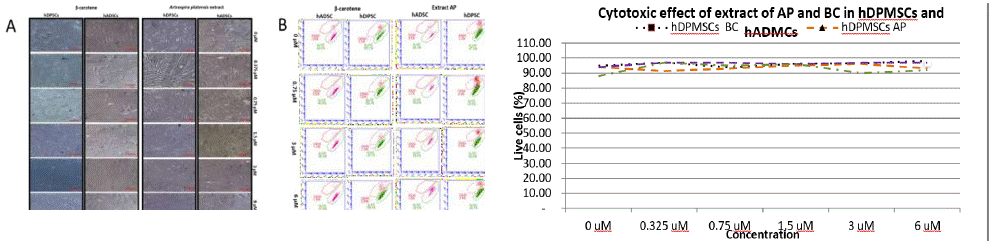
Figure 3: A) Monitoring by microscopy of cytotoxic effect of BC and AP extract in hDPMSCs and hADMSCs. The figure shows the maintenance of characteristic morphology of the hDPMSCs when exposed to both the AP extract or the BC after 72 hours. B) Dot Plots of the viability tests by cytometry of hMSCs treated with AP extract and BC showing 90 % viability. C) Viability tests of hMSCs treated with AP extract and BC showing 90 % viability.
This monitoring by microscopy is necessary to demonstrate evident morphological changes in the cells when exposed to a stimulus of the molecules in which it is not known or can cause cell death. Previous studies [21,22] demonstrate the need to have a first approach with microscopy follow-up which is the starting point to be able to give continuity in the trials to have evidence of cell viability with respect to its maintenance in adherence as well as the maintenance of its morphology [21].
Viability Analysis by Flow Cytometry
The cytotoxic analysis run by cytometry did not show cell death in the presence of the AP extract, Figure 3B.
Exposing the hDPMSCs to the extract and the standard showed that there is no negative impact on the maintenance of stability in terms of viability (Figure 3C). Several studies have looked for a cytotoxic activity, especially in studies of molecules where the antineoplastic potential was studied, however we did not find a cytotoxic effect in the concentrations used.
Differentiation to IPCs
The morphological changes are observed in the stage three, where the cells lose the fibroblastic morphology acquiring spherical shapes. These changes are mostly observed in the protocol where the AP extract was included during stage 3 and when it is added during all the three stages of differentiation being more visible in the hDPMSCs (Figure 4).
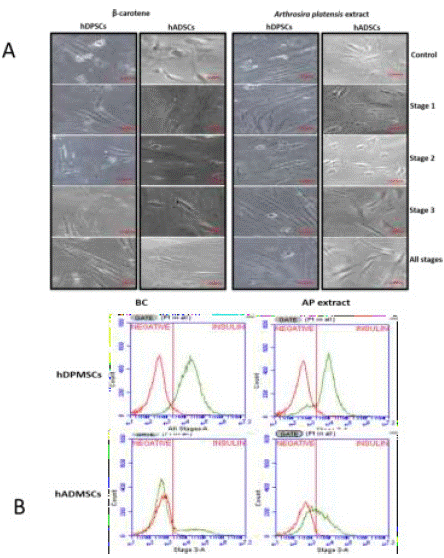
Figure 4: A) Differentiation to IPCs. In the image, the cellular morphology resulting from the differentiation of hMSCs is observed as the acquisition of a slight spherical morphology. B) Presence of intracellular insulin in hDPMSCs and hADMSCs where, with BC and AP extract at different stages of differentiation.
Reports of morphological changes after the differentiation of mesenchymal stromal cells into IPCs step by step where morphological changes are observed towards spherical cells such as cellular aggregation [23]. In our trials, cellular aggregation was not found, although rounding was observed since they eliminate the typical fibroblastic form on the other hand, other studies [24] mentioned that there are no significant morphological changes presented and similar morphological results found from our study. Other authors mentioned in general morphological changes with a tendency to form cellular aggregation which is different from our results.
Safer methods have been proposed by the addition in culture of growth factors, such as Growth Hormone (GH), Glucagonlike Peptide-1 (GLP-1) or Hepatocyte Growth Factor (HGF), but in human cells, the elevated proliferation is associated with a loss of IPCs features, like Pdx-1 or insulin expression [25]. Recently the generation of glucose responsive IPCs from hMSCs has been reported for the treatment of type 1 diabetes by Kim et al. 2012 [24]. But there are no reports on algal extract in cell differentiation method of hMSCs to IPCs.
Analysis of Intracellular Insulin
In our results, we found that adding AP extract or BC in the cellular differentiation process increases the presence of intracellular insulin in the differentiated cells in both hDPMSCs and hADMSCs. The percentage of differentiated cells that presents intracellular insulin in cells without submitting them to a treatment with either AP extract or BC is below 11.47%, contrasting with the differentiation assays where the extract or standard was added, where cellular differentiation percentages goes up to 70% (Table 2, Figure 4A, 4B). This was more outstanding in hDPMSCs that were stimulated with the BC in stage 3 of differentiation counting 74.36% of cells with the presence of intracellular insulin. When the AP extract was added to cell cultures at the second stage, 71.29% of cells showed the presence of intracellular insulin. In the case of the hADMSCs, a greater stimulus was presented when the AP extract was added both in stage tree and in all the stages presenting 48.45% and 25.65% respectively (Figure 5A, 5B).
Mesenchymal Stem Cell Type
Extract Type
Undifferentiated
Differentiated
Differentiated With AP or BC
Control
Without AP & BC
Stage-1
Stage-2
Stage-3
All
StagehDPMSCs
BC
0.80
0.71
2.32
5.76
38.13
74.36
AP
0.84
11.47
41.68
71.29
24.45
47.41
hADMSCs
BC
0.82
3.85
2.22
10.5
19.26
13.06
AP
0.81
5.43
8.65
17.87
48.45
25.65
Table 2: Percentages of intracellular insulin with the different treatments by adding the AP extract or the BC standard.
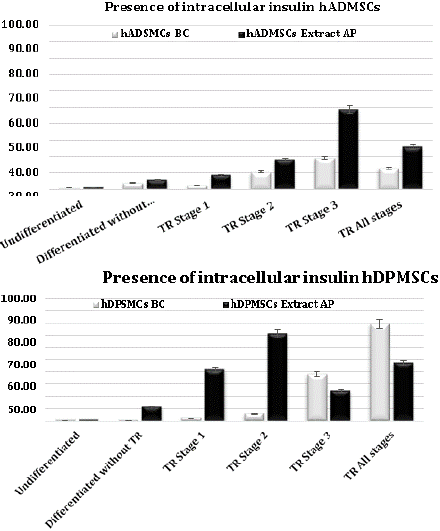
Figure 5: Percentage of differentiated cells. A) hADMSCs, showing intracellular insulin with AP extract and BC treatment. B) hDPMSCs, showing intracellular insulin production with AP extract and BC treatment.
In cell line hDPMSCs pure BC and AP extract both shown better intracellular insulin production at different stages of differentiation, whereas in hADMSCs cells AP extract shown better cell differentiation.
Several studies report their yield pancreatic islets with beta cells or insulin-producing cells by using anti-insulin antibodies generally by immunofluorescence techniques [26]. Other studies conduct this analysis by means of flow cytometry [27]. In this study we performed the cytometry analysis to quantify the cells that presented intracellular insulin as a starting point to verify if the extract or carotene induced this differentiation [28].The Kruskal-Wallis test was used. Since the P value is less than 0.05, there is a statistically significant difference between the all stages of application with undiffenciated cells with a level of 95.0% confidence (Figure 6).
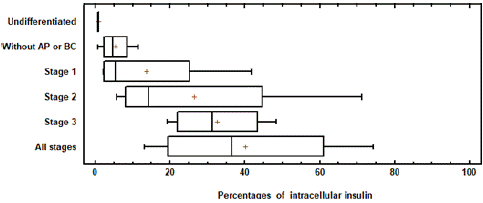
Figure 6: The Box Plot showing means of the different treatments. Percentage of differentiated cells. Showing intracellular insulin with AP extract and BC treatment in each stage of differentiation.
Analysis of GENE Expression
Typically, an up regulation in the expression of pancreatic endoderm markers as well as key transcription factors (Pdx-1, Nng-3, NeuroD, Pax-4, Nkx2.2, Nkx 6.1, Pax 6, Isl 1 and glucose transporter Glut-2) occurs during development of the pancreas. Pdx1 activates the promoters of several genes involved in the maturation of beta cells, including INS, GLUT2, glucokinase, and islet amyloid polypeptide. According to previous reports, glucose uptake in pancreatic beta cells is controlled by Glut-2, which is essential in the mechanism of glucose-induced insulin secretion [12].
The presence of PDX1 and NKX6.1 bands resulting from RT-PCR experiments, with-actin as an endogenous gene in the differentiated cells observed in our experiment, confirmed differentiation into beta cells, and the absence of the same genes was observed for the undifferentiated cells (Figure 7).
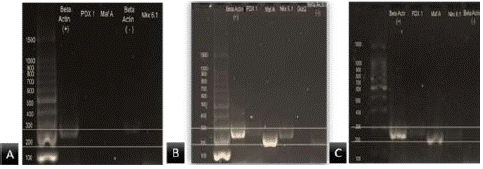
Figure 7: Analysis of gene expression. A) Shows just the expression of b-actin in hDPMSCs without differentiation. B) shows the expression of B-actin, Maf A, PDX1 and Nkx 6.1 in the differentiated hDPMSCs without the addition of AP extract or BC standard. C) Shows the expression of B-actin, MAFA, PDX1 and NKX6.1 in the differentiated hDPMSCs with the addition of AP extract in the stage two.
Similar to other studies [29,30] the goal was to obtain IPCs to analyze the presence of representative genes of β-cells. These studies mention that PDX1 is an important marker in the production of IPCs, and NKX6.1 is a gene that intervenes in the maturity of IPCs, with respect to the intervention of endocrine cells, but this is more evident in the second stage of differentiation due to which we perform the RT-PCR in mature cells, PDX1 and MAFA are the genes those were expressed (Figure 7).
Discussion
The use of cell replacements for different conditions or ailment is an area of intense research nowadays, in diabetes and specifically Diabetes Mellitus type 1 (T1DM). The purpose of such work is to repopulate the pancreas with insulin-producing cells obtained in vitro in order to replace the cell population lost [31]. One of the alternatives explored has been to obtain insulin-producing cells in vitro, by means of the differentiation of mesenchymal stem cells, thus avoiding the need of donors [32]. It is hoped that this cellular replacement will restore the normal function of the pancreas more efficiently than through therapies such as transplants, pharmacological therapies and treatments with recombinant proteins. Since these treatments have not shown sufficient efficacy and profitability to improve health conditions of the diabetic population and although β cells have already been obtained in vitro from human stem cells, a coarse yield in insulin production has not been found, to be proposed as candidates in cell therapy [33]. Therefore, the objective of the differentiation studies that have been carried out to analyze how the new approaches could be used to improve the performance and functionality of the pancreatic β cells obtained in vitro. The hMSCs are one of the pluripotent adult stem cells most relevant for these approaches, because of their ability to differentiate into replacement cells, modulate their local environment, activate endogenous progenitor cells, and secrete various factors. At least seven clinical trials are reported in phase I / II, where hMSCs exhibited exciting therapeutic effects [34]. However, a relevant point remains that is the requirement of a feasible and accessible source of hMSCs for the efficiency in the differentiation protocols. Si et al. 2012 reported especially cells from adipose tissue [35] and dental cells as a feasible source [1].
In addition to the above, the search for new molecules that induce the differentiation of stem cells takes relevance, although there is scarce information on the use of extracts of microalgae or bioactive molecules from those used in the differentiation of mesenchymal cells to IPCs. There are studies of the effects of BC in the differentiation of tumor cells by upregulation of differentiation markers, BC is a well-known antioxidant and precursor of Retinoic Acid (RA), and both induces differentiation [5]. It plays an important role as physiological process regulators such as embryonic development such is involved in cell differentiation and proliferation. Other studies also analyze the influence of BC on DNA methylation is very important process to maintain gene silencing in the development standard [36].
Studies about the use of biomolecules or extracts from microalgae specifically Arthrospira platensis and BC have been focused on evaluating their roles as possible therapeutic attributes like antioxidant activity. So, in this study, the impact of these molecules in the process of cell differentiation was explored. Fatty tissue mesenchymal trunks and in vitro dental pulp cells found that there is a need for stimulant to obtain better differentiation yields in the cells. With the presence of intracellular insulin, a low toxicity was evidenced, no evident morphological changes found when being added to use it in concentrations of 1.5 μM of BC and AP extract presenting a percentage of dead cells from 2% to 7%. Roosita et al. 2014, used concentrations of 5 μM in the trials, and report that there was no damage to the cell line. This is relevant to ensure that during the differentiation process the hMSCs will not be affected by exposure to phycoextracts.
These AP extract containing BC as well as the pure standard when added together with the mixture of molecules used in each of the stages of the differentiation process and throughout all the stages of differentiation showed morphological changes like the control of differentiated cells without adding AP extract or BC. These changes are observed until the end of the differentiation or from stage 3 in the differentiated hMSCs without adding the of AP extract or BC. In the process of differentiation of the hMSCs where segregate BC or AP extract morphological changes are observed from stage 1 of differentiation, being more evident in hADMSCs exposed to BC or AP extract. In the case of the hDPSCs, a change is shown when adding the AP extract, observing from stage 1 groupings by areas and until the end of the differentiation, similar to other reports [38].
The presence of intracellular insulin in both the hADMSCs and in the hDPMSCs that were exposed to BC, as well as in the AP extract, showed a higher population percentage of insulin-positive cells compared to the treatments in which they were not added. In particular, the highest percentages of intracellular insulin-positive cells without adding the extracts or BC reached 11%, whereas hDPMSCs reached up to 71% of cells with the presence of intracellular insulin when the AP extract was added. In the second stage of differentiation, a similar percentage was reached when adding BC throughout all stages of differentiation, for the case of the hADMSCs the highest percentage of cells with intracellular insulin presence was 48% when adding the extract in stage 3 of differentiation and 19% when adding BC in the same stage of differentiation so that the production of insulin in newly differentiated pancreatic β cells is favored from these mesenchymal stem cells when adding the treatments with AP extract or BC.
These results could be attributed to the antioxidant activity of both the BC and the molecules contained in the AP extract, this is discussed in other studies where they use molecules with antioxidant properties in an in vitro differentiation process such as [39,40], where they used astaxanthin in a protocol of differentiation of pluripotent cells induced towards the formation of neural progenitors, reporting the expression of the NeuroD gene, as well as inducing an increase in the levels of proteins related to cell proliferation and levels of expression of transcription factors related to the formation of neural progenitors. This is of relevance since it has been described that the NeuroD gene is involved in the maturation of beta cells during the secondary transition, NeuroD is activated in stage 2 of the process to act as a target of the Ngn3 gene which, as already described before, it is key to the development of endocrine cells. These results could be attributed to the antioxidant activity of both the BC and the molecules contained in the AP extract, this is discussed in other studies where they use molecules with antioxidant properties in an in vitro differentiation process such as [39,40], where they used astaxanthin in a protocol of differentiation of pluripotent cells induced towards the formation of neural progenitors, reporting the expression of the NeuroD gene, as well as inducing an increase in the levels of proteins related to cell proliferation and levels of expression of transcription factors related to the formation of neural progenitors. This is of relevance since it has been described that the NeuroD gene is involved in the maturation of beta cells during the secondary transition, NeuroD is activated in stage 2 of the process to act as a target of the Ngn3 gene which, as already described before, it is key to the development of endocrine cells [41].
The sample of the extract of Arthrospira platensis in stage 2 of the differentiation of hDPMSCs showed the bands of PDX1 NKX6.1 and MAFA, like the differentiated cells without adding the extracts. These genes are specific for beta IPCs, as several studies have shown amplifications for different genes such as PDX1, INS, Glut 2, Pax 6, Nkx 6.1, NKX6.2, Isl-1, with the objective of putting evidences that their cells differentiated towards pancreatic cells [42,43].
Conclusions
The biomolecules present in the phytoextracts of Arthrospira platensis and BC are promising not to alter the cell viability and to promote the differentiation of the hADMSCs and hDPMSCs, to IPC. Although the tests did not show significant morphological changes at the end of the differentiation assays, the presence of intracellular insulin demonstrated an increase in most of the treatments with respect to the differentiation process where they were not exposed to phytoextract or BC when applied to stage 3 and all the stages, presenting better results when using AP in the differentiation of hDPMSCs. The HPLC assay shown that the AP extract contains BC and another carotenoid pigment (probable Zeaxanthin/astaxanthin), so it may be due to interaction between these molecules, which causes favorable results regarding the presence of intracellular insulin.
Author Statements
Acknowledgments
We acknowledge the Council of National Science and Technology (CONACyT), Mexico for the financial support, PROJECT NO: 233146. Patricia Santibáñez. PH. D. Director of the department of maxillofacial surgery Hospital Civil of Guadalajara, Jalisco, México, who helped us for collection of dental pieces. QFB. David Macias, Undergraduate thesis, CIATEJ.
Conflict of Interest
The authors report no conflicts of interest in this work.
Funding
Supported by the National Council of Science Humanities and Technology (CONACHyT) (FOSSIS-No. 233146), México.
References
- Cañibano-Hernández A, Burgo LS, Espona-Noguera A, Ciriza J, Pedraz JL. Current advanced therapy cell-based medicinal products for type-1-diabetes treatment. Int J Pharm. 2018; 543: 107–20.
- Kornicka K, Houston J, Marycz K, Houston J. Dysfunction of Mesenchymal Stem Cells Isolated from Metabolic Syndrome and Type 2 Diabetic Patients as Result of Oxidative Stress and Autophagy may Limit Their Potential Therapeutic Use. 2018; 14:337-345.
- Aguayo-Mazzucato C, Bonner-Weir S. Pancreatic β Cell Regeneration as a Possible Therapy for Diabetes. Cell Metab enero de. 2018; 27: 57–67.
- Dassaye R, Naidoo S, Cerf ME. Transcription factor regulation of pancreatic organogenesis, differentiation and maturation. Islets. 2016; 8: 13–34.
- Lim JY, Kim YS, Kim KM, Min SJ, Kim Y. β-Carotene inhibits neuroblastoma tumorigenesis by regulating cell differentiation and cancer cell stemness. Biochem Biophys Res Commun. 2014; 450: 1475–80.
- Ma QY, Fang M, Zheng JH, Ren DF, Lu J. Optimised extraction of β-carotene from Spirulina platensis and hypoglycaemic effect in streptozotocin-induced diabetic mice. J Sci Food Agric. 2016; 96: 1783–9.
- Dey S, Rathod VK. Ultrasonics Sonochemistry Ultrasound assisted extraction of b - carotene from Spirulina platensis. Ultrason - Sonochemistry. 2013; 20: 271–6.
- Sadek KM, Lebda MA, Nasr SM, Shoukry M. Spirulina platensis prevents hyperglycemia in rats by modulating gluconeogenesis and apoptosis via modification of oxidative stress and MAPK-pathways. Biomed Pharmacother. 2017; 92: 1085–94.
- Cuantificación de carotenoides totales y β -caroteno en dos cepas de dunaliella salina (chlorophyta volvocales. Inst Boliv Ocean. 2008; 47: 67–76.
- Hejazi MA, De LC, Rocha JMS, Vermue M. Selective Extraction of Carotenoids from the Microalga Dunaliella salina with Retention of Viability. 2002; 79: 29-36.
- Karamzadeh R, Eslaminejad MB, Isolation AR. Characterization and Comparative Differentiation of Human Dental Pulp Stem Cells Derived from Permanent Teeth by Using Two Different Methods. J Vis Exp. 2012; 24: 4372.
- Chandra V, Swetha G, Muthyala S, Jaiswal AK, Bellare JR, Nair PD, et al. Islet-like cell aggregates generated from human adipose tissue derived stem cells ameliorate experimental diabetes in mice. PLoS One. 2011; 6: e20615.
- Park WS, Kim HJ, Li M, Lim DH, Kim J, Kwak SS. Two classes of pigments, carotenoids and c-phycocyanin, in spirulina powder and their antioxidant activities. Molecules. 2018; 23: 1–11.
- Paliwal C, Ghosh T, Bhayani K, Maurya R, Mishra S. Antioxidant, anti-nephrolithe activities and in vitro digestibility studies of three different cyanobacterial pigment extracts. Mar Drugs. 2015; 13: 5384–401.
- Hynstova V, Sterbova D, Klejdus B, Hedbavny J, Huska D, Adam V. Separation, identification and quantification of carotenoids and chlorophylls in dietary supplements containing Chlorella vulgaris and Spirulina platensis using High Performance Thin Layer Chromatography. J Pharm Biomed Anal. 2018; 148: 108–18.
- Lee HA, Park S, Kim Y. Effect of β-carotene on cancer cell stemness and differentiation in SK-N-BE(2)C neuroblastoma cells. Oncol Rep. 2013; 30: 1869–77.
- Putra A, Ridwan FB, Putridewi AI, Kustiyah AR, Wirastuti K, Anna N. The Role of TNF. a i nduced MSCs on Suppressive Inflammation by Increasing TGF- β and IL. 2018; 6: 1779–83.
- Vija L, Farge D, Gautier J-F, Vexiau P, Dumitrache C, Bourgarit A. Mesenchymal stem cells: Stem cell therapy perspectives for type 1 diabetes. Diabetes Metab. 2009; 35: 85–93.
- Trento C, Bernardo ME, Nagler A, Kuçi S, Bornhäuser M, Köhl U. Manufacturing Mesenchymal Stromal Cells for the Treatment of Graft-versus-Host Disease: A Survey among Centers Affiliated with the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2018; 24: 2365–70.
- Rolph DN, Deb M, Kanji S, Greene CJ, Das M, Joseph M. Ferutinin directs dental pulp-derived stem cells towards the osteogenic lineage by epigenetically regulating canonical Wnt signaling. Biochim Biophys Acta - Mol Basis Dis. 2018.
- Pauksch L, Hartmann S, Rohnke M, Szalay G, Alt V, Schnettler R. Biocompatibility of silver nanoparticles and silver ions in primary human mesenchymal stem cells and osteoblasts. Acta Biomater. 2014; 10: 439–49.
- Bertani N. Neurogenic potential of human mesenchymal stem cells revisited: analysis by immunostaining, time-lapse video and microarray. J Cell Sci. 2005; 118: 3925–36.
- Nekoei SM, Azarpira N, Sadeghi L, Kamalifar S. In vitro differentiation of human umbilical cord Wharton’s jelly mesenchymal stromal cells to insulin producing clusters. World J Clin Cases. 2015; 3: 640-9.
- Kim SJ, Choi YS, Ko ES, Lim SM, Lee CW, Il KD. Glucose-stimulated insulin secretion of various mesenchymal stem cells after insulin-producing cell differentiation. J Biosci Bioeng. 2012; 113: 771–7.
- Parnaud G, Bosco D, Berney T, Pattou F, Kerr-Conte J, Donath MY. Proliferation of sorted human and rat beta cells. Diabetologia. 2008; 51: 91–100.
- Abdulreda MH, Caicedo A, Berggren P-O. a Natural Body Window To Study Human Pancreatic Islet Cell Function and Survival. CellR4-- repair. Replace Regen reprogramming. 2013; 1: 111–22.
- Kobayashi N, Okazaki S, Sampetrean O, Irie J, Itoh H, Saya H. CD44 variant inhibits insulin secretion in pancreatic β cells by attenuating LAT1-mediated amino acid uptake. Sci Rep. 2018; 8: 1–10.
- Li L, Pan ZF, Huang X, Wu BW, Li T, Kang MX. Junctophilin 3 expresses in pancreatic beta cells and is required for glucose-stimulated insulin secretion. Cell Death Dis. 2016; 7: e2275.
- Samani FS, Ebrahimi M, Zandieh T, Khoshchehreh R, Eslaminejad MB, Aghdami N, et al. Vitro Differentiation of Human Umbilical Cord Blood CD133 + Cells into Insulin Producing Cells in Co-Culture with Rat Pancreatic Mesenchymal Stem Cells. 2015; 17: 211–20.
- Lynch K, Pei M. Age associated communication between cells and matrix: a potential impact on stem cell-based tissue regeneration strategies. Organogenesis. 2014; 10: 289–98.
- Abdulazeez SS. Diabetes treatment: A rapid review of the current and future scope of stem cell research. Saudi Pharm J. 2015; 23: 333–40.
- Zhang S, Dai H, Wan N, Moore Y, Dai Z. Promoting long-term survival of insulin- producing cell grafts that differentiate from adipose tissue-derived stem cells to cure type 1 diabetes. PLoS One. 2011; 6: e29706.
- Schiesser JV, Wells JM. Generation of β cells from human pluripotent stem cells: Are we there yet? Ann N Y Acad Sci. 2014; 1311: 124–37.
- Si Y, Zhao Y, Hao J, Liu J, Guo Y, Mu Y. Infusion of mesenchymal stem cells ameliorates hyperglycemia in type 2 diabetic rats: Identification of a novel role in improving insulin sensitivity. Diabetes. 2012; 61: 1616–25.
- Amer MG, Embaby AS, Karam RA, Amer MG. Role of adipose tissue derived stem cells differentiated into insulin producing cells in the treatment of type I diabetes mellitus. Gene. 2018; 654: 87–94.
- Kiec-Wilk B, Razny U, Mathers JC, Dembinska-Kiec A. DNA methylation, induced by beta-carotene and arachidonic acid, plays a regulatory role in the pro-angiogenic VEGF-receptor (KDR) gene expression in endothelial cells. J Physiol Pharmacol. 2009; 60: 49–53.
- Roosita K, Rimbawan SM, Djuwita I, Damanik MR, Kusharto CM, Damayanthi E. B-carotene roles in proliferation and differentiation, connexin and β-casein gene expression of mammary gland cells line. Malays J Nutr. 2014; 20: 113–9.
- RFX6 Regulates Insulin Secretion by Modulating Ca2+Homeostasis in Human 601 β Cells. Cell Rep. 2014; 9: 2206–18.
- Kim JH, Nam SW, Kim BW, Choi W, Lee JH, Kim WJ. Astaxanthin improves 603 stem cell potency via an increase in the proliferation of neural progenitor cells. 2010; 11: 5109–19.
- Kim JH, Nam SW, Kim BW, Kim WJ, Choi YH. Astaxanthin improves the proliferative capacity as well as the osteogenic and adipogenic differentiation 607 potential in neural stem cells. Food Chem Toxicol. 2010; 48: 1741–5.
- Shih HP, Wang A, Sander M. Pancreas Organogenesis: From Lineage Determination to Morphogenesis. Annu Rev Cell Dev Biol. 2013; 29: 81–105.
- Lee JS, An SY, Kwon IK, Heo JS. Transdifferentiation of human periodontal ligament stem cells into pancreatic cell lineage. Cell Biochem Funct. 2014; 32: 605–11.
- Pan G, Mu Y, Hou L, Liu J. Examining the therapeutic potential of various stem cell sources for differentiation into insulin-producing cells to treat diabetes. Ann Endocrinol. 2019; 80: 47-53.