
Research Article
Ann Agric Crop Sci. 2024; 9(2): 1152.
Comparative Analysis of Purification and Characterization Process of Phycocyanin Pigment from Spirulina Subsalsa with Arthrospira Platensis from Mexico
Bonilla-Ahumada FDJ; Khandual S*; García -Fajardo JA; Camacho-Ruíz RM; Padilla de la Rosa JD
Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco (CIATEJ), Unidad de Biotecnología Industrial, Mexico
*Corresponding author: Sanghamitra Khandual Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco (CIATEJ), Unidad de Biotecnología Industrial, Camino al Arenero #1227, Col, El Bajío Arenal, 45019 Zapopan, Jalisco, Mexico. Email: mita@ciatej.mx
Received: February 05, 2024 Accepted: March 14, 2024 Published: March 21, 2024
Abstract
The Arthrospira maxima, Spirulina major, and Spirulina subsalsa are plenty available in Mexican lakes and ponds. This cyanobacterium is used as an important dietary supplement due to its high protein content and vitamins. However, the most commercial strain in use is A. platensis, and very few reports exist on other Spirulina species from Mexico and their potential phycocyanin production. To use at a commercial scale, it is essential to evaluate native strains in an efficient purification process. In this work, we demonstrated the potential of Spirulina subsalsa for phycocyanin pigment production and purification process, compared with native A. platensis from Mexico. The biomass yield with S. subsalsa was 4.50±0.10 g/Lt, which was higher than A. platensis, which was 1.36 g/lt. The crude phycocyanin pigment from S. subsalsa was 267±0.37 mg/g, whereas 221±0.04mg/g in the case of A. platensis. After partial purification, we found a higher purity grade in S. subsalsa, with a higher yield and a recovery percent of 39.75%. In the case of A. platensis, after partial purification, the recovery percentage was 35.37. After ionic chromatography purification, the purity of phycocyanin was 2.57±0.03 in the case of S. subsalsa, whereas 1.91±0.02 in the case of A. platensis. SDS gel electrophoresis demonstrated two phycocyanin bands showing 17 kDa and 12 kDa in the case of both species. The two-step purification process functions very well for S. subsalsa species, whereas A. platensis demonstrated efficiency with one step. The extracted C-PC from S. Sulsalsa is safe as per the cytotoxicity assay.
Keywords: Spirulina; Cyanobacterium; Phycocyanin; Pigment extraction; Chromatography
Abbreviations: C-PC: Phycocyanin; CRUDEX: Crude Extract; AMS: Ammonium Sulfate; UF: Ultrafiltration; IEC: Ion Exchange Chromatography; PB: Phosphate Buffer; AP: Arthrospira Platensis; S.S: Spirulina Subsalsa; SDS-AGE: Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis; FTIR: Fourier Transform Infrared Spectroscopy Analysis; DIH2O: Distilled Water; DEAE-FF: Diethyle Aminoethyl Anion Exchange Column-Fast Flow.
Introduction
Photosynthetic cells are the most effective converters of solar energy into biomass for product manufacture. The total amount of annual global production of biomass is estimated to be as much as 12,000 metric tons, and more than half of that quantity is produced by China [1]. However, the commercial and industrial applications are limited due to the low quality of these refined products and productivity). Spirulina is the commercially known name of the two major strains: Arthrospira platensis and A. maxima (Bacteria, Cyanobacteria, Cyanophyceae, Oscillatoriales, Microcoleaceae, Arthrospira). However, there are other species of interest within the Spirulina genera, such as S. subsalsa (Z15), which is a promising source of phycocyanin. Recent studies implicated with enriched seawater medium the possibility of cultivating S. subsalsa, significantly reducing the cost for cultivation and increasing the profitable production of metabolites with commercial interest, such as phycocyanin [2]. Furthermore, S. subsalsa strain possesses a higher growth rate and higher phycocyanin (C-PC) yield compared with the commonly studied A. platensis [3]. We isolated the S. subsalsa strain from the fish-pond Jocotepec, Chapala area, located in the western part of Mexico. These cyanobacteria are gram-negative prokaryotic planktonic cells found worldwide in fresh and marine waters [4]. This type of alga is an important diet supplement and has been used as a source of protein and vitamins in humans since ancient times [5]. It is rich in protein (up to 70%), it also contains vitamins such as B12 and provitamin A (β-carotenes), and it produces phycocyanin (20% dry weight), which is a pigment with potent antioxidant activity. It is also rich in phenolic acids, tocopherols, and Gamma-Linolenic Acid (GLA) [6].
Phycocyanin (PCY) is a bioactive compound that has enormous demand in the global market of US$245.5 million, estimated by 2027 Meticulous research [7]. This is reported after the coronavirus pandemic analysis disease 2019 (COVID-19), and considered as a powder or aqueous forms, also demanded according to the grade of purity (analytical, cosmetic, or food grades), and by applications like (biomedical, diagnostics, beverages, foods, nutraceuticals and pharmaceuticals), and geographical demands by [7] Meticulous research which cannot be underestimated. C-PC has so many humanitarian health benefits as it acts as an antioxidant, anticancer, anticarcinogenic, anti-inflammatory, neuroprotective, hepatoprotective, immunomodulatory, and reno-protective pharmacological effects and also antidiabetic potential and non-toxicity towards human health [8,9,].
Cyanobacteria are the only prokaryotes that perform oxygenic photosynthesis with Phycobilisomes (PBS), which is a photosynthetic pigment apparatus. They contain Phycobiliproteins (PBP), among them Phycocyanin (C-PC), falls into one of the four groups [10]. It has two polypeptide subunits (a and β) that form an oligomeric peptide called P.C. covalently linked to chromophores with up to 160–180 amino acid residues in a and β subunits. The molecular weight of a and β subunits nearly from 10–19 kD and 14–21 kD, respectively, depending on species, and the ratio of a and β subunits is usually 1:1 [11]. Purification and separation are very important to reach the highest extraction yield with purity, for which we need to prevent the degradation of the product and reduce operation costs [12]. Most of the methods reported before for the purification of C-PC consist of several steps that generate high costs, and loss of biological activity during the multi-step purification process is a matter of concern [13]. Moreover, the growing demand for green extraction procedures and purification processes leads to adopting sustainable technologies that can be used for the manufacture of products like fine chemicals, pharmaceuticals, biofuels, and protein-rich biomass at the same time for a variety of applications.
Our study aims to assess the qualitative and quantitative content of phycocyanin in S. subsalsa species in comparison to A. platensis, both collected in Mexico, to explore their biotechnological potential. The partial purification processes, like one-step and two-step ammonium sulfate precipitation, were also compared with phosphate buffer and water as solvents using the sonication process for cell breakage. Again, purification with the omission of the ammonium sulfate step is also intended to evaluate the purity grade, quantity, and recovery efficiency of C-PC to see if the process can be used for easy industrial scale-up without affecting purity grade. Purification with Ion-chromatography resulted in S. subsalsa species being a promising source of phycocyanin with high purity and quantity to use as an analytical grade and develop industrial products. This study compared the quantity and quality of phycocyanin extracted by different purification methods, like solvents, ammonium sulfate precipitation or without precipitation step, membrane filtration, chromatography, and as well as characterization by SDS of C-PC from two spirulina species. The present study focuses on the importance of using green technologies to eliminate conventional solvents like phosphate buffer to generate a less expensive purification process and an alternative new source of phycocyanin production from species like S. subsalsa.
Materials and Methods
Biomass Cultivation
Spirulina subsalsa strain was isolated from a fish tank, jocotepec, near Chapala Lake, estimated as Mexico’s largest lake in Jalisco. The cultivation was done using Zarrouk’s medium in a photoautotrophic 7L (Applikon) bioreactor with constant aeration and 24-hour LED illumination to promote phycocyanin pigment production (C-PC). After 25 days, the biomass was collected by centrifugation at 10 min 10,000 rpm (Sorvall) and dried in an oven at 60°C, 24 Hrs. Once completely dried, the biomass was ground to obtain a fine powder. The commercial Arthrospira platensis biomass was obtained from the Biomex industry, thoroughly dried, and used as a fine powder to compare the extraction between the native isolated strains.
Phycocyanin Extraction & Estimation
The Phycocyanin (C-PC) extraction was performed following the [14] protocol with the Arthrospira platensis and Spirulina subsalsa strains using a 1:100 ratio consisting of 1 g dried biomass and 100 mL of distilled water (DIH2O) and two types of phosphate buffers as solvents (0.1 M) 5.1 pH and 7.5 pH with 30 min sonication (ultrasonic SB5200DTN) to disrupt the cell wall and overnight cold-water agitation to release the Phycocyanin (C-PC). The resulting blueish solution was centrifugated for 10 min at 6,000 rpm (Eppendorf 5810R) to retrieve the crude C-PC extraction, and the resultant biomass was used for further applications such as metabolite extraction procedures for fatty acid compounds, etc.
Cytotoxicity Assay
The Artemia franciscana was used as a bio-model to perform the cytotoxicity test for S. subsalsa extract. There was a control culture with no crude extract and three different concentrations of crude extract: 100 ul (0.5%), 200 uL (1%), and 500 uL (2.5%); added into 20 mL of prepared artificial seawater (45 g/L) filtered with 0.45 cellulose membrane and used to start a population of 10 (n=10) with Artemia. A. Franciscana cultured each one in triplicate to evaluate cytotoxicity with critical response in the first 24 hours of exposure to the aqueous crude extract of phycocyanin using the protocol from [4]. After the first 24 hours, the experiment was continued until a week period to confirm any toxic compound in the extract was going to affect the Artemia franciscana species.
Partial Purification
Partial phycocyanin purification was achieved using a hypersaturated solution of Ammonium Sulfate (AMS) where 76.79 g (NH4)2SO4 were weighed per 100 mL distilled water (DIH2O). Subsequently, it is stirred with a hot plate at 450 rpm, applying 100°C of heat to increase the colligative properties of the solvent to favor the complete dissolution. Afterward, the AMS solution was used to promote Phycocyanin (C-PC) precipitation, a one-step process by using 60% (v/v) AMS only, and the other two-step process as follows.
In the two-step process, first a concentration of AMS 20% (v/v) was used that was poured in, then kept overnight to precipitate and discard undesired proteins by centrifugation 10 min 6,000 rpm (Eppendorf 5810R), then the supernatant was recovered and a second application of AMS 55% (v/v) was carried out and let the solution kept overnight at 40 C. Later, it was centrifugated for 10 min at 6,000 rpm to concentrate the partially purified phycocyanin and then resuspended in DIH2O for further proceedings [15].
Partial Purification by Ultracentrifugation
After ammonium sulfate precipitation the concentrated phycocyanin was resuspended in DIH2O and then ultra-centrifugated for 10 min 10,000 rpm (Biorad) using 50 mL falcon tubes (cornning) with 50 kD membrane filter integrated to wash the AMS residue and concentrate the phycocyanin where the phycocyanin and its subunits obtained in the upper part of the membrane is located and the salts with other undesired compounds, escaped from the membrane during centrifugation.
Ion-Exchange Chromatography Purification (IEC)
The concentrated phycocyanin above was resuspended in distilled water (DIH2O), then equilibrated in phosphate buffer 0.1 M, and then purified through the Ionic Protein Liquid Chromatography using a DEAE FF-cellulose (Cytiva) column previously equilibrated with phosphate buffer 0.1 M to perform the ion-exchange purification. The equilibrated phycocyanin (5 mL) was injected into the column and was eluted by different phosphate buffer elution solutions with incremental NaCl concentrations (0- 0.5 M) to promote ion exchange interactions according to Safeei et al. [16] method. Samples after chromatography and kept in refrigeration to compare results and SDS-PAGE analysis.
Fourier Transform Infrared Spectroscopy analysis (FTIR)
The FT-IR studies have been followed by the method described by Davis and Mauer [17]. The 10 mg of lyophilized C-PC samples from all the types of extracts like STD (sigma) sample, crude extract, UFC extract, and IEC purified were subjected to an FTIR spectrophotometer (model- Cary 630 FTIR) to produce the I.R. spectrum. I.R. spectra region 400-4500 cm-1 were recorded at room temperature 25°C. For each spectrum, the frequencies for all sharp bands were accurate to 0.01 cm-1
SDS-PAGE
The electrophoresis was made by taking 10 ul concentrated samples collected from the different extractions and purified from the chromatography column using the two strains. One reactive grade phycocyanin standard was obtained from Sigma-Aldrich was used as a control (Sigma-P2172) and was compared to confirm the phycocyanin bands present in two native spirulina species using the sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) at 12% concentration running with a (Sigma-P2172) molecular marker 14-40 kD during 180 min, with 100 V according to the Walls [18] protocol.
Results and Discussion
Biomass Quantification
After 30 days of the culture, the biomass yield of Spirulina subsalsa was 4.50±0.10 g/Lt with an average of 0.15 g/Lt biomass growth per day. This report is quite interesting regarding biomass yield quantity nearly four times previously reported. There are only a few previous reports regarding Spirulina subsalsa yield exhibited biomass production around one g/Lt as reported newly [3] for its biotechnological potential for phycocyanin production using a low-cost seawater medium. Here, the major challenges associated with C-PC production include cultivation cost reduction, alternative strain with a higher yield, and C-PC extraction efficiency with a cost-effective purification process without using toxic solvents.
As Arthrospira platensis is a commercial strain for phycocyanin production at present, reported by several authors with an amplified range of biomass yield, like Díaz et al. [19], reported 0.3 g/Lt maximal daily biomass productivity using Guillard’s f/2 medium. Karemore et al., (2020), using Zarrouk’s medium, demonstrated a wide range of daily growth ratios depending on the culture conditions that encompasses 0.18 – 0.25 g/Lt in A. platensis [20] cultivated A. platensis using the same Zarrouk medium and reported a yield of 2.92 g/Lt, noticeable lower quantity compared with the present research indicating that this native Spirulina subsalsa (S.S.) represents a promising and highly productive strain. Another advantage, according to Selvendran [21], is that one of the advantages of Spirulina cultures is that they are much less susceptible to contamination due to high alkalinity in the medium or sea water medium, ensuing the result of clean cultured Spirulina biomass for cost-effective applications.
Cytotoxicity Assessment
The Artemia franciscana exposure to C-PC survival after seven days is represented in Figure 1. The control without any extract at the end of the 168 hours with only 3±1 living crustaceans survived. The next groups showed better survival rates with a high concentration of C-PC exposure. With the 0.5% extract, we obtained 6±2, 1% extract 8±2, and 2.5% extract 10±2 living organisms after 168 hours of exposure to C-PC, respectively. The result demonstrated better survival in the treated samples as probably the C-PC presence in the medium was the only protein source or food available, and that did not exist in the control group. Therefore, the results confirmed that the C-PC extracted from Spirulina sub salsa is safe and does not contain any harmful cyanotoxins that negatively affect other living organisms.
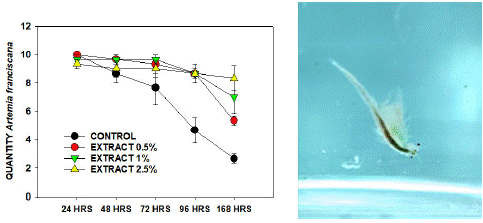
Figure 1: Artemia franciscana survival rate after seven days of C-PC exposure with different concentrations: 0.5%, 1%, 2.5%, and control without any extract. C-PC exposure curve confirming the non-toxicity (A). A. franciscana with C-PC present in the medium (B).
Phycocyanin Yield and Partial Purification
The phycocyanin (C-PC) extractions and yield from Arthrospira Platensis (A.P.) and Spirulina subsalsa (S.S.) are represented in Table 1. The first stage of the extraction was with only cold distilled water (DIH2O) in case of A.P., we obtained 221±0.04 mg/g of C-PC (22.1% of biomass) with a purity ratio of 0.34±0.07 and in the case of S.S. higher quantity of phycocyanin was achieved that is 267±0.37 mg/g (26.7 % of biomass) with a purity ratio of 0.33±0.03. The quantity of C-PC was significantly higher with S.S. Therefore, all the downstream processes with S.S. have greater C-PC quantity compared with A.P. However, the purity ratio is nearly the same for both.
The precipitation process was done by the ammonium sulfate method. During the one-step precipitation process with AMS 65% done using DIH2O as a solvent for crude extract, we found 49.89±0.17 mg/g C-PC with 1.05±0.02 purity ratio for A.P. and 110.77±0.26 mg/g C-PC with 0.93±0.01 purity ratio for S.S. S.S. here shown almost 2.22 times higher phycocyanin production with double recovery rates like 44.36 % and 22.57% from the crude extract in the case of A.P. Using the phosphate buffer as extraction solvent with pH 5.1 (PB5), we found 39.15±0.12 mg/g (C-PC) with 1.06±0.05 purity, and phosphate buffer with pH 7.5 (PB7), we found 78.18±0.16 mg/g (C-PC) with 1.02±0.08 purity in case of A. platensis. But with S.subsalsa strain using PB5, we obtained 103.73±0.30 mg/g of C-PC with 0.90±0.01 purity; using PB7, we got 129.28±0.80 mg/g (C-PC) with 0.99±0.01 purity for S.S. Here, we can say phosphate buffer works better for partial purification with a step process at 7.5pH, which was also demonstrated by other workers (Table 1). The recovery percentage was 35% in the case of A.P. and 48% in the case of S.S.
In the two-step process, after discarding the first precipitated part with AMS 20%, the rest extract again precipitated with AMS 55% when DIH2O used as an extraction solvent showed 10.19±0.03 mg/g C-PC yield with 0.94±0.05 purity for A.P. and 125.98±0.41 mg/g C-PC yield with 1.15±0.01 purity for S.S. In this case, we found the step extraction process gives a significantly higher yield in the case of S. subsalsa with a food-grade purity. With PB5, the C-PC yield was 6.89±0.04 mg/g containing a purity value of 0.77±0.04 and with PB7, 5.10±0.0mg/g containing a purity value of 0.87±0.04 for A.P. But in the case of A.P., a two-step process is not a good choice, even if with buffer, it has shown less yield. In the case of using PB5, we got C-PC yield of 129.84±0.63mg/g with 1.06±0.03 purity, and using PB7 yield was 106.47±0.17 mg/g with 1.19±0.06 purity for S.S. (Figure 2, Table 1). It’s quite impressive results in case of S.S. we can get C-PC almost 12% of biomass after AMS precipitation with food grade purity.
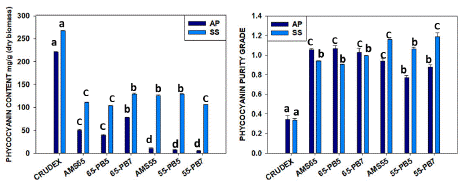
Figure 2: Comparative yield by the one-step precipitation process (AMS 65%) and two-step precipitation process (AMS20%+ 55%) for quantity and purity C-PC from Arthrospira platensis and Spirulina subsalsa. (similar letters represents no difference among treatments whereas different letters shows significant difference statistically.
The results demonstrate that AMS 65% for both the strains had more C-PC recovery compared with AMS 55% due to the high salt availability that produced more precipitation, as shown in Figure 3. Nonetheless, the purity ratio has no significant difference between the AMS 65% and AMS 55%. The recovery percentage was found to be better, like 35% and 48%, in both the strains using one-step precipitation with AMS65%, as in two-step precipitation, only S. subsalsa showed better results in the percentage of recovery, like 39-47%. Specifically, for A.P., the AMS 65%-PB7 had the better outcome for quantity and purity. Meanwhile, S.S. had the best extraction result using AMS 55%-DIH2O for quantity and purity. This implies that precipitation incrementing AMS only affects the amount of recovered C-PC and has no significant effect on partial purification. Hence, depending on the strain, the amount of C-PC has considerable variation. Clearly, S.subsasa has shown good yield, like 12.9% concentrated C-PC after partial purification and 47% recovery with a 1.15 purity value, which can be used as food additives extracted with water only (Table-1, Figure 2). Here, the comparison between phosphate buffer and water used for crude phycocyanin extract was clear. After ammonium sulfate precipitation, it was clearer whether one can use a one-step process or two steps for better purity, which can depend on strains or species. C-PC is considered to be food grade when >0.7, cosmetic grade when >1.5, reactive grade when > 3.9, and analytical grade when >04.0 when the purity ratio is like this range, respectively [22-24].
Figure 3: One-step (AMS 65%) and two-step precipitation process of Phycocyanin (20%+ AMS 55%) for comparative yield and quality C-PC. Analysis. AP: Arthrospira Platensis and SS: Spirulina Subsalsa.
Our results are higher than the previous; that is, total C-PC content was lower in A. platensis and S. sub salsa at 6.4% and 7.5%, respectively, with purity no higher than 0.7 observed [3]. However, there are reports of very similar or higher percentages of recovery using the AMS precipitation method [24] which found that the ASP procedure was very effective in increasing the recovery of C-PC. AS concentration is critical to determine if it will be effective on the yield and purity index [22]. Gorgech et al. [12] reported a significant increase in C-PC yield upon using higher AS concentrations, like 60%, with 92% recovery of PCY [12]. In another report, using AMS at 65% resulted in 53% of C-PC yield [25]. But purity value varies in all the cases. Spirulina platensis C-PC yield was reported before but not analyzed much after the one-step and two-step process or comparative analysis with phosphate buffer and water as a solvent (Table-1, Figure 3).
Purified Phycocyanin by Ultrafiltration (U.F.)
After AMS precipitation, partially purified phycocyanin (C-PC) passed through ultracentrifugation (U.F.) with both the strains, Arthrospira Platensis (A.P.) and Spirulina Sub salsa (S.S.), and the results shown in Table 2 and C-PC recovery interpreted from all the different treatments based on the crude extract type (CREX).
After Ultracentrifugation (U.F.) with the samples from the one-step process (AMS 65%) with DIH2O C-PC yield was 39.54±0.17 mg/g with 1.13±0.01 purity ratio for A.P. and 95.73±0.07 mg/g with 1.08±0.02 purity ratio for S.S. which is more than double the amount. Using the Phosphate Buffer 5.1 pH (PB5) samples for ultracentrifugation (U.F.), the yield was 28.30±0.12 mg/g (c-Pc) with 1.10±0.03 purity and by using phosphate buffer 7.5 pH (PB7) previous sample, the C-PC was 69.67±0.20 mg/g with 1.15±0.01 purity respectively for A.P. In the case of S.S., PB5 samples yielded 84.99±0.04 mg/g C-PC with 1.04±0.03 purity, and PB7 samples demonstrated 78.68±0.03 mg/g C-PC yield with 1.28±0.02 purity. In the case of S. sub salsa, both water and PB7 were found suitable to concentrate C-PC quantity (7.8-9.5%), the recovery rate was found [29-35] after ultracentrifugation (U.F.), and purity had similar values. (Table-2, Figure 4)
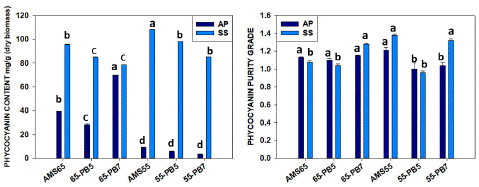
Figure 4: Comparison one-step process (AMS 65%) and two-step process (AMS 55%) for quantity and purity C-PC ratio between Arthrospira platensis and Spirulina subsalsa after ultracentrifugation. (Similar letters represents no difference among treatments whereas different letters show significant difference statistically.
The previous samples with a two-step process (AMS 55%) using DIH2O after ultracentrifugation (U.F.) showed 9.26±0.03 mg/g C-PC yield and 1.21±0.05 purity for A.P., which was a very low yield in comparison to S. subsalsa where we found 108.31±0.03 mg/g C-PC with 1.38±0.02 purity almost 10 times higher. By using PB5, previous partially concentrated samples after ultracentrifugation (U.F.), C-PC content was 5.98±0.04 mg/g with 1.06±0.12 purity, and by using PB7, C-PC content was 3.36±0.08 mg/g with 1.04±0.06 purity for A.P. But in the case of S. subsalsa with PB5 samples after ultracentrifugation (U.F.), we got 98.08±0.02 mg/g C-PC with 1.00±0.06 purity, and by using PB7, C-PC content was 85.16±0.04 mg/g with 1.32±0.05 purity for S.S. (Table 2, Figure 4) with is a significant result not reported before.
We suggest a two-step extraction process with water is the best method for not spending for phosphate buffer with notable recovery percent after U.F. that is 31-40%. As in the case of A. platensis, the single-step process was good, with a recovery rate of 17-31 % after U.F. without much-affecting purity. Nevertheless, A.P. with AMS 65%-PB7 has 69.67±0.20 mg/g as better C-PC recovery and 1.21±0.05 purity ratio achieved with AMS 55%-DIH2O after U.F.
The one-step process (AMS 65%) has no intermediate actions; meanwhile, the two-step process includes precipitation with (AMS 20%) first, then the second step with AMS 55% during the precipitation. All the treatments with different letters present statistically significant differences between them.
C-PC purification is usually done using precipitation and chromatographic techniques [26,27]. The purity grade or Purity Index (P.I.) of C-PC can be determined by dividing absorbance at 620nm with absorbance at 280nm. Here, we could achieve C-PC purity of nearly 1.5 after ultracentrifugation with 50KD membrane, and it is of cosmetic grade, according to previous reports [22,24,27]. Although AMS is a widely used method for C-PC purification, eliminating the salt after precipitation is time-consuming and challenging. Recently, direct ultrafiltration was reported by various workers like Singh et al. [28] who obtained a purity index of 2.89 for C-PC, and the recovery was 83.58% without using AMS.García-L´opez et al. [29] also achieved a purity index of 2.65 and 3.72 by Membrane filtration and U.F., using A. maxima biomass. Likewise, Sala et al. (2018) used the U.F. process for C-PC concentration with 50 kDa, and the y recovery of C-PC was 93.4% with a purity index >1.5, which can be used for cosmetic purposes. But in our case, we could achieve nearly 1.5 purity grade using the AMS precipitation step and then Ultracentrifugation. Further analysis was done by modifying the process of ultracentrifugation, eliminating the AMS step, as the number of studies reporting C-PC purification by membrane techniques is limited in the literature. Again, M.F. and U.F. techniques are easy to scale up for large-scale operations for industrial applications without washing steps.
Phycocyanin Purified by Ion-Exchange Chromatography
The Purified phycocyanin (C-PC) by Ion-Exchange Chromatography (IEC) obtained with Arthrospira Platensis (A.P.) and Spirulina Subsalsa (S.S.) are demonstrated in Table 3 and Fig-5. The one-step process samples with DIH2O for A. plantensis and the two-step process with DIH2O for S.subsalsa selected from ultracentrifugation process to proceed for further purification with ionic exchange chromatography because they resulted the highest C-PC purity ratio . One-step process samples (DIH2O) showed 3.09±0.12 mg/g C-PC yield with 1.91±0.02 purity ratio for A.P. and 25.15±0.04 mg/g with 2.57±0.03 purity ratio for S.S. AMS 55%-DIH2O obtained 0.59±0.17 mg/g and 1.60±0.01 purity for A.P. and 21.53±0.03 mg/g with 2.45±0.02 purity for S.S. as it was is demonstrated in Figure 5. Once again, during the ion exchange chromatography purification, a considerable amount of C-PC was lost, and the recovery percentage was 8-9% in S. subsalsa. In the case of A. platensis, it was very low, like 1.4%. Nonetheless, results with purity ratio have no statistically significant difference among the one-step and two-step processes in the case of S. subsalsa, indicating that the presence of NaCl (0.3 M) during the ion interactions has the same effect whether the extracted C-PC had higher or lower AMS concentrations during the precipitation.
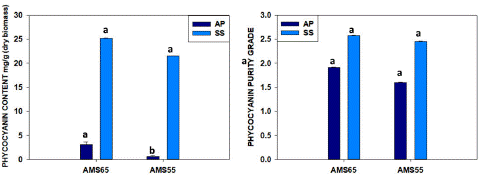
Figure 5: Comparison one-step process ANS 65% and Two-step AMS samples after IEC purification A) C-PC quantity and B) C-PC purity from A. platensis and S. subsalsa. (similar letters represents no difference among treatments whereas different letters show significant difference statistically).
There are several chromatographic methods like Ion Exchange Chromatography (IEC), Gel Filtration Chromatography (GFC), Membrane Chromatography (MC), and hydrophobic interaction (membrane) chromatography (HIC/ HIMC) were reported for the purification of C-PC [13,22,23] and among them IEC method is more common due to higher yield of C-PC with analytical grade. However, there are reports on elution buffers with pH gradients [30] reported a purity value of 1.82, and recovery was 77.3% at pH 7.5 at room temperature. But Bermejo et al. [31] improved the C-PC purity index, P.I.2.0 from S. platensis with a recovery of 80%, using an anion exchange matrix, gel filtration matrix, and Sephadex G-100. Different combinations of techniques gave better results, like C-PC purification from S. platensis, which reported a maxim purity index of 3.9 in the case of ultrafiltration followed by IEC with pH gradient [24,27]. reported that Arthrospira platensis C-PC purification by IEC using salt gradient elution with NaCl 0.3M. and obtained C-PC 1.68 purity. However, it was not clear in those previous reports about the recovery percentage calculated from the previous ultracentrifuged sample or from the crude extract.
Apart from that, there is no report related to Spirulina subsalsa purification, and thus, here we report C-PC 2.4-2.6 purity value after the purification by IEC NaCl 0.3M. So here we tried using a cation exchange column (Cytiva-DEAE-FF) and achieved purity P.I.2.0 from S.subsalsa, but we found that the recovery percentage from the crude extract was 8-9%. But in the case of platensis, we could not achieve good results like recovery was very low 1.4%, which may be due to the biomass purchased being stored for a long.
Phycocyanin Quality Analysis by FTIR
IR spectroscopy is an accepted tool for the characterization of biomolecules [32]. This spectrometer uses a blackbody radiator as an Infrared (I.R.) photon source. Infrared studies are multidisciplinary, but more attention is paid to biochemical investigation. The FT-IR technique was used to evaluate the type of organic and inorganic complexes in plants. Phytochemical constituents of Spirulina by FTIR method had very few reports and were recorded in the region of FT-IR spectra in 3428-3320 cm-1 to 620-490 cm-1 in the different frequency ranges by [33] but with whole algae powder. Still, not many reports on phycocyanin FTIR signature have been reported yet. The FTIR spectrum of the extract of Ulva lactuca reported phycocyanin as the most bioactive component with a transmittance maximum of 1652.
So, to compare all the types of extracts, we subjected to FTIR analysis and found same pattern of the picks with all. As the purity increases with UCF and IEC samples the pick height was higher. Again, all the extracts had a similar pattern to the C-Pc standard we purchased from Sigma Aldrich. (Figure 6, Table 4). Here we found phycocyanin as the most bioactive component with transmittance maximum peals at 1000, 1038, 1519,1680, 2989, and 3255 in the case of S. sub salsa and A. platensis; both were very much similar to our Phycocyanin standard purchased from sigma. So, purity level can be trusted as much as the Sigma standard.
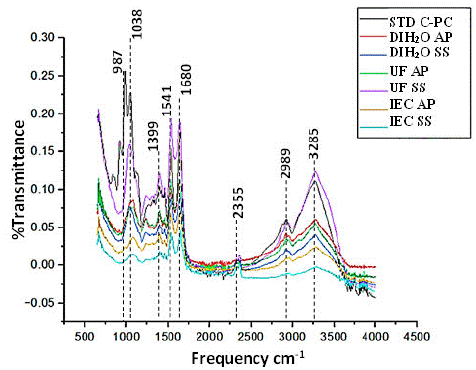
Figure 6: FTIR spectrum for phycocyanin extract analysis from S.subsalsa Y A. platensis.
Characterizing the C-PC by FTIR spectroscopy method is a rapid, simple, and nondestructive technique that can give evidence for the presence of various functional groups. S. platensis C-PC demonstrated functional groups with the frequency of peaks 674 and 795 cm-1, of which 1456, 1539, and 2359 cm- 1 were S–O stretching vibration, CH2 bending vibration, the protein amide II band and presence of carboxylic acids, respectively [25]. Patel A. et al. (34) reported that the protein amide II band was detected at 1539 cm- 1 (C– –O stretching) and the sharp amide I band at 1655 cm -1 from the I.R. spectra of freeze-dried C-PC of Spirulina, Phormidium, and Lyngbya spp.
Phycocyanin Characterization by SDS-PAGE
The C-PC extractions of Arthrospira Platensis (A.P.) and Spirulna Subsalsa (S.S.) at the final steps of Ultracentrifugation (50kD) (UTC) and Ion-Exchange Chromatography (IEC) were put together in a sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) to demonstrate the presence of the protein bands with the extracted samples as it is shown in figure 6. In all cases, the C-PC is evident as the a and β subunits are migrated within the 21kDa and 14kDa range compared with the C-PC standard used as a control sample (sigma) as an indicator and also with a protein marker (Sigma) with band size. Furthermore, the high purity ratio C-PC obtained the highest absorbance signal, it is shown (Figure 7-B) in the spectral scanning, with the spectrophotometer having the I.C. bands as the highest pick among all and the crude extract as the lowest at 620nm signal. These results confirm that the purification processes are effective for both strains A.P. and S.S. for C-PC as they increase the absorbances according to their purity level, and consequently, the undesired compounds are removed.
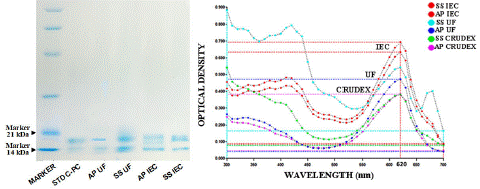
Figure 7: SDS-PAGE reveals the C-PC from the ultrafiltration (U.F. 50 kDa) and ion-exchange chromatography (IEC): (A) lane 1- molecular marker, lane-2-C-PC standard(sigma), lane-3-AP UFC sample, lane-4-SS UFC sample, lane-5-AP IEC sample, lane-6-SS IEC sample. (B) different purity level of C-PC samples pick height at 620nm absorbance during the spectral scanning.
In previous reports, characterization of C-PC from Limnothrix sp. also demonstrated maximum absorbance spectra at 620 nm and fluorescence at 644 nm [35]. The molecular mass of C-PC was reported to be between 81 and 215 kDa in different cyanobacterial genera, and a and β subunits were found between 15.2 and 24.4 kDa [15,34]. In Calothrix sp., two subunits with molecular weights of 17 and 21 kDa using SDS-PAGE (M.C. Santiago-Santos et al. 2004) were found. Patel et al. [34] reported the molecular weight of β subunit for all C-PCs that was 24.4 kDa. Still, the molecular weights of a subunits vary in different cyanobacteria like 17, 19.1 and 15.2 kDa for Spirulina, Phormidium, and Lyngbya spp., respectively. However, in the case of S. subsalsa, there were no reports regarding a and β subunits. We found very similar band sizes like 14kDa and 17kDa for a and β subunits like S. platensis Standard purchased from Sigma (P2172).
Conclusions
The Spirulina subsalsa resulted as a promising and alternative source of C-PC when compared with commercial strains like S. platensis, usually considered for biotechnological purposes such as metabolite production and pigment accumulation. Each and every step of the yield and recovery percentage was evaluated comparatively when precipitation, filtration, and chromatographic purification steps were done to predict which process would be more efficient and which stain is better for C-PC yield with more purity. In this work, we generated evidence to suggest that S.subsalsa is a robust and precious strain, mainly to produce C-PC that can be used for industrial purposes. Furthermore, we characterized the C-PC subunits are very similar to A. platensis by SDS bands. The extract is also nontoxic, and with the efficient purification method, the product can achieve high purity with the IEC method with the value of C-PC enhanced to 2.4, suitable for the cosmetic grade or more quantity with the UCF method for the food industry.
Author Statements
Acknowledgments
The authors are grateful to CONACHYT for their financial support as a fellowship to the students and to the Laboratory of Industrial Biotechnology CIATEJ for the infrastructure provided during this investigation.
References
- Masojídek J, Torzillo G. Mass Cultivation of Freshwater Microalgae. In: Encyclopedia of Ecology, Five-Volume Set. Elsevier Inc. 2008; 2226–35.
- Bezerra PQM, Moraes L, Cardoso LG, Druzian JI, Morais MG, Nunes IL, et al. Spirulina sp. LEB 18 cultivation in seawater and reduced nutrients: Bioprocess strategy for increasing carbohydrates in biomass. Bioresource Technology. 2020; 316: 123883.
- Jiang L, Yu S, Chen H, Pei H. Enhanced phycocyanin production from Spirulina subsalsa via freshwater and marine cultivation with optimized light source and temperature. Bioresource Technology. 2023; 378: 129009.
- Ruebhart DR, Cock IE, Shaw GR. Brine shrimp bioassay: Importance of correct taxonomic identification of Artemia (Anostraca) species. Environmental Toxicology. 2008; 23: 555–60.
- Jubie S, Dhanabal SP. Gas Chromatography-Mass Spectrometry Analysis and Antibacterial Activity of Fatty acid Mixture of Spirulina platensis. 2012; 4: 1836-1838.
- Sajilata MG, Singhal RS, Kamat MY. The Carotenoid Pigment Zeaxanthin-A Review. 2008; 7: 29-49.
- Phycocyanin Market by Form (Powder, Liquid), Grade (Food Grade Phycocyanin, Cosmetic Grade Phycocyanin, Reagent and Analytical Grade Phycocyanin), Application (Food and Beverages, Pharmaceuticals and Nutraceuticals), Geography - Global Forecast to 2030.
- Ou Y, Lin L, Yang X, Pan Q, Cheng X. Antidiabetic potential of phycocyanin: Effects on KKAy mice. Pharmaceutical Biology. 2013; 51: 539–44.
- Gupta M, Dwivedi UN, Khandelwal S. C-Phycocyanin: An effective protective agent against thymic atrophy by tributyltin. Toxicology Letters. 2011; 204: 2–11.
- Stadnichuk VI, Lukashev EP, Yanyushin MF, Zlenko D V., Muronez EM, Stadnichuk IN, et al. Energy transfer pathways among phycobilin chromophores and fluorescence emission spectra of the phycobilisome core at 293 and 77 K. Doklady Biochemistry and Biophysics. 2015; 465: 401–5.
- Jiang L, Wang Y, Yin Q, Liu G, Liu H, Huang Y, et al. Phycocyanin: A potential drug for cancer treatment. Journal of Cancer. 2017; 8: 3416–29.
- Gorgich M, Passos MLC, Mata TM, Martins AA, Saraiva MLMFS, Caetano NS. Enhancing extraction and purification of phycocyanin from Arthrospira sp. with lower energy consumption. Energy Reports. 2020; 6: 312–8.
- Kumar K, Dasgupta CN, Das D. Cell growth kinetics of Chlorella sorokiniana and nutritional values of its biomass. Bioresource Technology. 2014; 167: 358–66.
- Khandual S, Sanchez EOL, Andrews HE, de la Rosa JDP. Phycocyanin content and nutritional profile of Arthrospira platensis from Mexico: efficient extraction process and stability evaluation of phycocyanin. BMC Chemistry. 2021; 15.
- Soni B, Kalavadia B, Trivedi U, Madamwar D. Extraction, purification and characterization of phycocyanin from Oscillatoria quadripunctulata-Isolated from the rocky shores of Bet-Dwarka, Gujarat, India. Process Biochemistry. 2006; 41: 2017–23.
- Safaei M, Maleki H, Soleimanpour H, Norouzy A, Zahiri HS, Vali H, et al. Development of a novel method for the purification of C-phycocyanin pigment from a local cyanobacterial strain Limnothrix sp. NS01 and evaluation of its anticancer properties. Scientific Reports. 2019; 9: 9474.
- Davis R, Davis R, Mauer LJ. Fourier Transform Infrared (FT-IR) Spectroscopy: A Rapid Tool for Detection and Analysis of Foodborne Pathogenic Bacteria Fourier tansform infrared (FT-IR) spectroscopy: A rapid tool for detection and analysis of foodborne pathogenic bacteria. 2010; 1582-1594.
- Walls D, Loughran Editors ST. Protein Chromatography Methods and Protocols Second Edition Methods in Molecular Biology 1485.
- Díaz JP, Inostroza C, Acién FG. Scale-up of a Fibonacci-Type Photobioreactor for the Production of Dunaliella salina. Applied Biochemistry and Biotechnology. 2021; 193: 188–204.
- Ramanathan N. Comparative studies on production of Spirulina platensis on the standard and newly formulated alternative medium. 2013; 1.
- Algal Bioree nery: An Integrated Approach Debabrata Das Editor.
- Lauceri R, Chini Zittelli G, Torzillo G. A simple method for rapid purification of phycobiliproteins from Arthrospira platensis and Porphyridium cruentum biomass. Algal Research. 2019; 44: 101685.
- Liu P, Choi JW, Lee MK, Choi YH, Nam TJ. Spirulina protein promotes skin wound repair in a mouse model of full-thickness dermal excisional wound. International Journal of Molecular Medicine. 2020; 46: 351–9.
- Moraes CC, Sala L, da Costa Ores J, Braga ARC, Costa JAV, Kalil SJ. Expanded and fixed bed ion exchange chromatography for the recovery of C-phycocyanin in a single step by using lysed cells. Canadian Journal of Chemical Engineering. 2015; 93: 111–5.
- Prabakaran G, Sampathkumar P, Kavisri M, Moovendhan M. Extraction and characterization of phycocyanin from Spirulina platensis and evaluation of its anticancer, antidiabetic and antiinflammatory effect. International Journal of Biological Macromolecules. 2020; 153: 256–63.
- Chen G, Cai Y, Su Y, Gao B, Wu H, Cheng J. Effects of Spirulina algae as a feed supplement on nutritional value and flavour components of silkie hens eggs. Journal of Animal Physiology and Animal Nutrition. 2019; 103: 1408–17.
- Amarante MCA de, Braga ARC, Sala L, Moraes CC, Kalil SJ. Design strategies for C-phycocyanin purification: Process influence on purity grade. Separation and Purification Technology. 2020; 252: 117453.
- Singh NK, Parmar A, Madamwar D. Optimization of medium components for increased production of C-phycocyanin from Phormidium ceylanicum and its purification by single step process. Bioresource Technology. 2009; 100: 1663–9.
- García-López DA, Olguín EJ, González-Portela RE, Sánchez-Galván G, De Philippis R, Lovitt RW, et al. A novel two-phase bioprocess for the production of Arthrospira (Spirulina) maxima LJGR1 at pilot plant scale during different seasons and for phycocyanin induction under controlled conditions. Bioresource Technology. 2020; 298: 122548.
- Silveira ST, De Menezes Quines LK, Burkert CAV, Kalil SJ. Separation of phycocyanin from Spirulina platensis using ion exchange chromatography. Bioprocess and Biosystems Engineering. 2008; 31: 477–82.
- Bermejo R, Ramos A. Pilot scale recovery of phycocyanin from spirulina platensis using expanded bed adsorption chromatography. Chromatographia. 2012; 75: 195–204.
- Quinteiro Rodriguez MP. Clinical Microbiology Newsletter Fourier Transform Infrared (FTIR) Technology for the Identification of Organisms. 2000; 22: 57-61.
- Theivandran G, Ibrahim SM, Murugan M. Fourier Transform Infrared (Ft-Ir) Spectroscopic Analysis of Spirulina Fusiformis. Vol. 3, ~ 30 ~ Journal of Medicinal Plants Studies. 2015; 3: 30-32.
- Patel A, Mishra S, Pawar R, Ghosh PK. Purification and characterization of C-Phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expression and Purification. 2005; 40: 248–55.
- Gantar M, Simovic D, Djilas S, Gonzalez WW, Miksovska J. Isolation, characterization and antioxidative activity of C-phycocyanin from Limnothrix sp. strain 37-2-1. Journal of Biotechnology. 2012; 159: 21–6.