
Research Article
Ann Agric Crop Sci. 2023; 8(3): 1135.
Flocculation and Cell Rupture in C. Vulgaris
Khandual S¹*; Santiago-Mateo H¹; Maldonado-Ortiz AJ¹; Bonilla-Ahumada FJ¹; Kumar JS²
1Department of Industrial Biotechnology, The Center for Research and Assistance in Technology and Design of the State of Jalisco A.C (CIATEJ), México
2Department of Civil & Environmental Engineering, Old Dominion University, USA
*Corresponding author: Khandual S Department of Industrial Biotechnology, The Center for Research and Assistance in Technology and Design of the State of Jalisco A.C (CIATEJ), Normalistas 800 Ave, Colinas de La Normal, 44270 Guadalajara, Jalisco, México Email: mita@ciatej.mx
Received: April 27, 2023 Accepted: May 26, 2023 Published: June 02, 2023
Abstract
Among all carotenoids, the human body can easily convert β-carotene into retinol, which is a major ingredient in dietary supplements. In the United States, beta-carotene (95% pure) is permitted as a colorant under 21 CFR 73.95 and 21 CFR 166.110. In this study, we are reporting the evaluation of the content of lipids, β-carotene and lutein in the native strain of Chlorella vulgaris CIB 46 (from CIBNOR), Mexico during growth period to define the optimal harvest days for the specific products and future industrial use. This strain has the potential to produce lutein, β-carotene, lipids that can be used for functional foods. Chlorella vulgaris CIB 46 strain showed considerably high biomass productivity reaching almost 2.4g/L biomass on day 21 and reaching 3.0g/L on Day 24 in photobioreactors. Chlorella vulgaris presented a production of carotenoids up to 3.45mg/g at day 21. The Thin-Layer Chromatography (TLC) showed that they contain high β-carotene and lutein in their carotenoid fraction based on the intensity of the band. Our study results showed that Chlorella vulgaris-CIB46 is a potential strain to produce main carotenes such as β-carotene and lutein, they are 0.35% of dry biomass. We obtained a notable amount of lipids that is 37% in Chlorella vulgaris CIB 46 measured by sulpho-valiline method of extraction with enzymatic pretreatment with enzyme Celluclast at 2%. Lipid content was high at 15 days of culture whereas we found β-carotene was higher at 15th and 21st day.
Key words: Biomass; Carotenoids; Thin-layer chromatography; Beta-carotene; Lutein
Introduction
Currently microalgae importance increased many folds for its use as feed for aquatic and terrestrial animals for the nutritional value and includes use as colorant in aquaculture, and high-protein or polyunsaturated fatty acid supplement in human diets. The food, pharmaceutical and cosmetic markets have an enormous growing trend for microalgae-based products [1]. Furthermore, the large number of existing species of microalgae constitutes a large biodiversity, which has a scope for potential commercial exploitation for many novel products besides vitamins, pigments, and polyunsaturated fatty acids [2,3]. The key factor for their eventual economic feasibility is the possibility of good selected species, estimating their biomass production rate and metabolites producing algae to use it for industrial scale production [4,5]. The most popular microalgae source of carotenoids are Chlorella, Chlamydomonas, Dunaliella, Muriellopsis and Haematococcus sp. all of which belong to the Chlorophyceae family [1]. They tend to accumulate carotenoids and thus offering alternatives to chemical synthesis [6]. Among all natural sources Dunaliella is the most studied and commercially popular strain used for beta-carotene production, the highest content of 9-cis β-carotene [7,8] reaching up to 100g/kgDW, [9-11]. But it required high salt concentration and biomass production is less in comparison to other algal strains. Although many microalgae can produce carotenoids, most of them have not been reported with scientific data regarding pigment yield and their bioprocess for efficient extraction.
Here we intended to work on a Chorella vulgaris CIB46 strain from Mexico with the processes like cell wall breakage efficiency and harvesting to evaluate the feasibility of the strain to explore its carotenoid and lipid production for future use at an industrial scale. Using fresh water native algae as an alternative source of beta-carotene and lutein production with cheaper culture media has an advantage over saltwater algae Dunalliela salina. It gives an idea regarding alternative source of Beta-carotene and Lutein production from Chlorella vulgaris with autoflocculation and specific mechanical or enzymatic pretreatment processes method which does not includes toxic chemical use.
Materials and Methods
Algal Culture by Airlift Bioreactor
In this work algal culture done by aeriation with 200mL/min in an airlift bioreactor, without pH control at the room temperature. We observe growth and biomass by optical density at 680nm at 3 days interval up to 21 days. We evaluated biomass for extraction of lutein and lipids during the day 12-21 days. Culture used for the dry biomass, centrifuged at 4000rpm for 15 min at 25°C and kept them in the oven at 60°C for 48 hours.
Methods of Extraction and Estimation of Lipids
For lipid extraction, we used the method of Byreddy et al. [12] with wet biomass. We carry out a wash with 25mL of distilled water (dH2O) resuspending the biomass and shaking slightly manually. After centrifuging at 4,000rpm for 10 min at 25°C, the water was discarded, 5mL of KOH 5% in methanol (CH3-OH) was added to the biomass, stirred homogeneously and left to stand for 15 min to remove pigments. Then the biomass collected after centrifuge and cell wall breakage done by sonication for 10-15 minutes. We did the extraction with 3 different solvent combinations to compare lipid extraction efficiencies to determine the right solvent to use. Water was added and centrifuged to separate organic phase and then proceed to lipid quantification by sulfophospho-vanillin method.
Solvents used:
a) Method 1. Chloroform–methanol 2:1
b) Method 2. Chloroform–methanol 1:1
c) Method 3. Ethanol-Hexane 2.5:1
Lipid Estimation by Spectrophotometry
The lipid estimation was carried out by the method of Mishra et al. [13] using Sulfo-Phospho-Vanillin (SPV). With this method it was possible to make the direct quantitative measurement of lipids within a culture. SPV reacts with lipids to produce a distinct pink color, and its intensity can be quantified using spectrophotometric methods for absorbance measurement at 530nm. We made a standard curve with vegetable oil for the interpretation of the amount of lipids extracted in microalgae.
The solvent was completely evaporated. We added 100μL of deionized water (diH2O) to each tube, then added 1mL of concentrated sulfuric acid (H2SO4 97-99%) and shaken with extreme care. The tube was placed in an oven at 100°C for 15 minutes, then removed from the oven and immediately placed in ice for 5 min. Then 1mL of (SPV) was added, shaken homogeneously and then the tubes were placed in an incubator at 36-37°C for 15 minutes. The tubes were shaken and measured in triplicate in an EON BioTek spectrophotometer at a wave length of 530nm.
Method of Extraction and Estimation of Carotenoids
Extraction using different solvents
First step we quantify lutein content by different types of solvents to consider which solvent is better suitable for this strain. We compare hexane, acetone, ethanol, methanol, and tetrahydrofuran. In the case of solvents, we found acetone and ethanol were with higher extraction yields of Lutein. Methanol was also good for extraction, but due to toxic nature to use in the food sector it was avoided. The quantification was with spectrometry method and TLC for quality analysis in extracts.
Carotenoid saponification and estimation
In the present study the method of Rajashree Hajare et al. [14] was used for carotenoid saponification and extraction. We extracted 200mL of Chlorella vulgaris culture to obtain wet biomass by centrifugation. The biomass samples were dried in an oven at 60°C for 48 hours. Grinded dry biomass added with 1mL of absolute ethanol, transferred to the glass tubes and sonicated for 30 min at 25°C. Then 20mL of absolute ethanol was added, placed in an oven at 50°C for 20 minutes and then removed to keep them at room temperature. Then 50mL KOH at 4% and 6mL of Hexane was added. Then the samples were left to stand overnight (18 hours) in the absence of light, centrifuged at 7000 rpm for 15 min to separate the hexane phase. Then proceeded for optical density measurement in a BIOTEK brand EON spectrophotometer at 448 and 446nm. The remaining sample was dissolved in the appropriate amount of solvent so that it could be mounted on TLC to see qualitative analysis.
To determine the concentration of lutein we use the following formula: Lut=[(A)(V)(Fd)]/(€xW). Where: Lut=Concentration of lutein (μg/g biomass), A=absorbance at 446 nm, V=volume of the extract in mL, Fd=Factor of dilution, €=Coefficient of the absorption (2589), W=dry biomass (g).
Enzymatic method
Acetate Buffer (ACB) was prepared and adjusted the pH to 5.5. For the rupture of the cell wall, the enzymatic hydrolysis of the cellulose was carried out, through treatments where the conditions were varied according to the supplier's instructions. Novozyme celluclast enzyme was used with acetate buffer at pH 5.5 with an incubation temperature of 55°C. With the same buffer, the Trichoderma cellulase enzyme (SIGMA) was used at pH 5 in 55°C incubation. The hydrolysis conditions were standardized with the same culture medium without adjusting the pH to determine if it was effective for cell degradation without consuming another type of buffer with different pH. We used 10mL of Chlorella vulgaris culture concentrate, transferred to Eppendorf 200μL of sample in duplicate, the simples were considered like: Control 0, Control C1 (thermal treatment), T1 (thermal-enzymatic treatment-in BBM medium), T2 (treatment thermal-enzymatic- in acetate buffer). For samples T1 and T2 (x4 of each sample), centrifugation is carried out at 2000rpm for 1 min, the supernatant liquid is discarded and 196μL of BBM medium are added for T1 and acetate buffer for T2. The samples were placed in the Thermo-shaker Brand: Benchmark, (samples C1, T1, T2) at 55°C at 300rpm for 3.5 hours. After 3.5 hours, a Neubauer cell count was performed to evaluate the percentage of live and dead cells with this treatment.
Results
Biomass Yield
This strain shown considerably higher biomass production reaching almost 2.4g/L biomass on day 21 and reaching 3.0g/L. Day 24 with airlift culture condition. Zheng et al., [15] reported that in the aerated culture of Chlorella vulgaris a biomass concentration of approximately 3.28g/L was obtained, and a lipid productivity of 35%. In our case we also found similar results with respect to biomass production with aeration.
Flocculation Study for Effective Harvest
Various reports have shown that Chlorella does not present auto-flocculation, however, in our case we did observe auto-flocculation in 12 hours of darkness with 82% flocculated bio mass. The settled biomass was remarkable, this implies good results to avoid the use of so many chemicals. We also compared flocculation efficiencies by using chemicals to compare previous reports.
The results shown with Ca (OH)2 treatment, 71% biomass was flocculated in 5 min and 82% biomass flocculated in 2 hours with 28% volume of solution added. With other reagents, we did not found flocculation immediately, but after 2 hours we found 57-81% flocculated biomass with spending 60% reagent by volume which is not recommendable (Table 1 & Figure 1). An alkaline treatment with Ca (OH)2 is recommended for flocculation and at the same time serves to break the cell wall for the extraction of biofuel and other compounds. Auto flocculation presented better results than the other flocculation treatments.
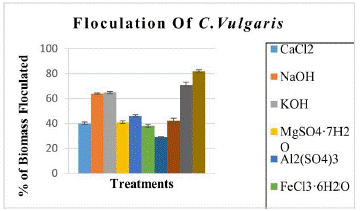
Figure 1: % of flocculated biomass by different methods.
Reactivo
Floculation %-5Min
Floculation % 2hr
Floculation %-4hr
% Of reactive use v/v
pH
CaCl2
40±4.53
53±0.55
61±1.14
60
6.5
NaOH
64±0.52
75±2.87
81±1.86
60
13.9
KOH
65±0.55
76±1.25
81±0.61
60
14.2
MgSO4·7H2O
41±0.91
57±3.51
67±1.56
60
7.1
Al2(SO4)3
46±1.03
64±5.15
65±1.17
60
2.8
FeCl3·6H2O
38±1.08
60±4.57
75±11.92
60
2.0
Fe2(SO4)3·6H2O
29±0.27
61±5.46
72±8.05
60
1.7
FeSO4·7H2O
42±1.39
81±0.50
84±1.05
60
3.9
Ca(OH)2
71±2.06
82±0,16
88±1.89
28
12.5
Autofloculation 12 horas
NA
NA
82±0
0
8.0
Table 1: Biomass flocculation by different treatments.
Diffrent Cell Rupture Methods
Methods
Treatments
Samples
% of Cell death
Chemical
Acids
Control 0
0
HCl
49±0
H2SO4
60±0.86
H3PO4
28±2.42
HNO3
24±0.51
Bases
Control 0
0
NaOH pH11
28±0.57
NaOH pH12
46±0.57
Physical
Autoclave
Control 0
0
15 min
30±3.29
30 min
31±0.98
Microwave
Control 0
0
5 min
28±5.6
10 min
46±0.63
Sonication
Control 0
0
10 min
38±3.0
20 min
45±2.65
30 min
47±2.34
Enzymatic
Cellulase
Control 0
0%
C1
55±3.29
T1(BBM)
41±0.63
T2(ACB)
47±2.13
Celluclast
Control 0
0
C1
55±3.29
T1(BBM)
64±0.28
T2(ACB)
73±0.28
Table 2: Cell death percentage by different cell rupture methods.
SOLVENT
Lutein CHO (mg/g)
Hexane
0.121±.002
Acetone
9.797±.003
Ethanol
9.214±.003
Methanol
9.059±.004
Hydrotetrafuran
9.041±.003
Table 3: Lutein contents (mg/g biomass) with different solvents.
The cost and energy demand of microalgae for harvesting could be significantly reduce if the cells could be pre-concentrated by flocculation. During flocculation, individual cells form larger aggregates that can be separated from the medium by simple gravity settling. There are many methods to flocculate algae, high pH levels can cause the precipitation of calcium or magnesium salts [16]. Since the salts rearranges at higher pH ranges as they are non-ionic, the crystal structure. In magnesium hydroxide, divalent magnesium cations are replaced by trivalent iron or aluminum. Despite the circumstances, there is a neutralization of charges. Negative algae cells are attracted by positive salt compounds. The only addition would be the cations, anions, or entire compounds necessary to prepare appropriate salts. Charge neutralization can be achieved by lowering the pH. Once there are diminished electrostatic forces acting on the cells, they can be separated easily out of solution by gravity. However, extreme pH can be detrimental to algae health. Other methods include the use of bio flocculants. They are very reliable for commercial uses such as wastewater treatment and mineral processing. Although metallic salts are being applied for the harvesting of microalgae, e.g. Dunaliella [7], their use results in high concentrations of metals in the harvested biomass that remain in the residue.
Flocculants based on natural biopolymers are a safer alternative. In order to interact with the negative surface charge of microalgal cells, these biopolymers must have a positive charge, which is rare in nature. A well-known positively charged biopolymer is chitosan, which is a very efficient flocculant but works only at low pH, but the pH in microalgae cultures is relatively high [17]. Flocculation often occurs spontaneously in microalgae cultures when the pH increases [18]. This type of flocculation is called autoflocculation because it occurs spontaneously in microalgae cultures because of an increase in pH due to depletion of photosynthetic CO2 (Figure 2). In our case, we found auto flocculation in our Chlorella vulgaris cultures.

Figure 2: Flocculation in Chlorella vulgaris cib 46.
Cell Rupture Methods
Breaking down cell walls is a time and energy consuming process; it is vital to maximize lipid extraction efficiency. Therefore, it must be ensured that the selected strategy produces cell rupture. However, due to variations in microalgae cell wall chemical composition, cell size, and morphology, the optimal method of cell wall disruption is species-specific and must be determined for each strain, separately. Various approaches have been used to break down the cell walls, including mechanical, chemical, and enzymatic methods. Different methods of cell rupture processes with C. vulgaris-CIB46 are analyzed here, considering their advantages and disadvantages. Normal methods of cell disruption by mechanical disruption can serve to further degrade or denature the desired products [19-21]. Thermal degradation of triglycerides produces several compounds, many of which cannot be converted to biodiesel. So here in our study several cell disruption methods including enzymatic ones were evaluated to better understand which method is more suitable for lipid extraction. Here we found efficient 55 to 73% cell breakage by celluclast enzyme, then 60% by 1% sulfuric acid treatment.
Lipid Extraction Results with Dry and Wet Biomass
It was shown that the best performance for extraction and to obtain a good amount of lipids is using solvents such as chloroform and methanol at a 1:1 ratio. When quantified, 11.66±0.26mg/L of lipids was obtained in Chlorella vulgaris CIB46. To determine the necessary amounts of solvent to be used, we decided to compare the use of chloroform and methanol at a 2:1 ratio with which 8.368±1.08mg/L lipid was obtained (Figure 3). In order to not to spend too much solvent it was the 1:1 ratio of Chloroform-Methanol was recommended. Chloroform was chosen since in our case we were getting a little more quantity of the lipid, than in ethanol: hexane combination. Although in the case of ethanol: hexane it was easy to evaporate the solvents and they are less toxic. In addition, it was observed that there was more lipid content (Figure 4) when extracted with wet biomass, in our case twice the amount compared to dry biomass (Figure 4). In the case of dry biomass, the amount of lipid was much lower between 3.61±0.26mg/L, there was probably less cell wall rupture, so as not to waste energy on drying biomass, we recommended for the wet biomass method.
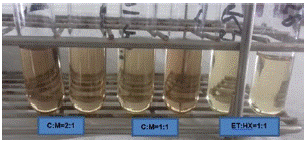
Figure 3: Lipid estimation of C. vulgaris mg/lt by SPV method.

Figure 4: Lipid Content of C. vulgaris mg/lt by different solvents.
Lipid Yield during the Exponential Growth Phase of Chlorella Vulgaris
We first compared different solvents with suitable methods for lipid extraction from Chlorella vulgaris with wet and dry biomass to determine which solvent is most suitable. In this case we determined that the solvent Chloroform: Methanol 1:1 ratio showed higher lipid extraction in wet biomass with sonication. But due to the presence of pigments, it is advisable to wash with KOH. We also compared the production of lipids in the airlift bioreactor, which showed a range of 69-370mg/g, almost 7-37% of lipids in biomass during growth (Figure 5). On day 15th we achieved a greater amount of lipid.

Figure 5: Lipid Content (mg/g biomass) of C. vulgaris cib 46 during growth period.
Microalgae are known for their high lipid content (20% to 50% dry weight) and high growth rates, being a promising oil source for biodiesel production [22,23]. However, the production of biodiesel from microalgae lipids is not economically feasible, as it requires several optimizations to reduce production costs and meet the current demand for biofuels [24,25]. Algae oil extraction is an expensive process that can determine the sustainability of algae-based biodiesel. [26] reported a lipid content of 24.85 to 36.45%, Yessica I. et al. [27] reported 52 to 58%, Liang et al. [28] reported 38% lipid content and Chia et al. [29] reported a lipid content of 33.5% in Chlorella vulgaris. But in our case, we also obtained a notable amount of lipids 37% (Figure 5) in Chlorella vulgaris CIB46. It is very similar to previous reports. We still improve the most efficient pretreatment processes to achieve a greater amount of lipids, which facilitates cell wall rupture for better extraction. The SPV method (Figure 3) is actually very sensitive in measuring the nanogram level of lipid quantitation. The use of colorimetric/spectrophotometric method for the rapid detection of microalgal lipids constitutes a promising and accurate technique to determine the productivity of microalgal lipids.
Extraction of Lutein with Different Solvents
In the case of Chlorella vulgaris, we achieved a notable amount of lutein between 9.04-9.7mg/g in a 21-day culture. Using TLC method, we observed that they still had more chlorophyll and other carotenoid extracts (Figure 6). For these reasons we proceeded to saponification method to obtain Lutein with higher purity. In this case, the high content of pigments is probably due to interference from other pigments apart from Lutein that are represented in our TLC (Figure 6 left).
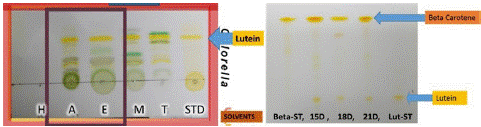
Figure 6: Extraction of lutein with different solvents (left) and extraction of lutein after saponification (right).
When the extraction of lutein between dry and wet biomass was compared, a higher yield of lutein was observed in the wet biomass with 0.77% compared to the dry biomass 0.75%. That is why it is more feasible to use wet biomass for lutein extraction.
Lutein Saponification and Evaluation
Chlorella vulgaris presented a production of 1.48mg/g at 15th day, 1.46mg/g at 18th day and 3.45mg/g of lutein at day 21. This is the main factor that determines the best yield performance of the molecule of interest, to easily reach its biological potential. As we can see on TLC, they contain lutein in their carotenoid fraction. However, based on the intensity of the band, the highest concentration of carotenoids we found are beta-carotene and lutein as well, but lesser amounts (Figure 6 right). This is an extract that comes together with beta-carotene, in future processes it can be separated by preparative column separation.
Discussion
The cell wall composition of a microalgae species can affect the process of extraction, making it easier or harder to access its valuable contents. Presence of high levels of polysaccharides in the cell wall, such as glucose and mannose, present in Chlorella zofingiensis, or by complex sugars composition such as arabinose, galactose, rhamnose, mannose and xylose, as found in Tetraselmis suecia and T. striata makes it difficult to extract bioactive compounds efficiently. Algaenan or sporopollein is another extremely resistant biopolymer, a non-hydrolyzable biopolymer, composed of long ω-hydroxy fatty acids chains linked by several types of chemical bond, found in some species such as Chlorella spp., Nannochloropsis galditana and Scenedesmus spp. But prokaryotic algae like Arthorspira spp. cell wals contain peptidoglycan, which makes it less rigid and susceptible to breakage [30]. Thus, several methods applied here to breakdown the cell wall of Chlorella vulgaris Cib46 a native strain from Mexico in this, present study and confer the enzymatic rupture is efficient for valuable compounds like lutein and lipid rich in PUFA extraction efficiently and environment friendly. Recently ozone was used as an alternative approach to harvest and disrupt microalgae Chlorella vulgaris for biodiesel production [31] as most of the reports says algal harvesting is difficult and an energy consuming process. But here we found with alkali treatments 75 to 82% flocculation within two hours and auto flocculation (82%) was also possible keeping the biomass in dark which is very economic process and without much salt contamination in biomass. As there are very few reports about Mexican native strains of microalgal bioactive compounds, it will serve as natural resource utilization by local industries to commercialize the valuable products.
Conclusion
Biomass harvesting in Chlorella Vulgaris can be carried out by auto flocculation. The methods appreciated for the extraction of lipids basically consist of using wet biomass, using KOH for the removal of pigments, extraction with solvent Chloroform- Methanol 1:1. Chlorella vulgaris-CIB46 is a potential strain for lipid production with 37% dry biomass. With pretreatments methods, that are H2SO4 and Celluclast, they show greater rupture of the cell wall, improving the yield in the extraction with solvent Chloroform-Methanol 1:1. For lutein extraction, it was found that it is more efficient to use moist biomass with ethanol and hexane as solvent. Chlorella vulgaris-CIB46 is a potential strain for lutein production with 0.3-0.4% dry biomass, also presence of Beta-carotene was found prominently in TLC data that has to be qualified and separated in next work. Thus, the extraction of lutein and lipids from Chlorella vulgaris CIB-46 from Mexico, for their incorporation into the food sector as a natural colorant in beverages and other industrial uses also look promising. Currently, the use of microalgae has an important impact in the field for human consumption as a source of antioxidants, leaving this work as a background for the efficient extraction method of flocculation and cell rupture to extract lipids and lutein efficiently from Chlorella vulgaris.
Author Statements
Acknowledgments
We are thankful to the Mexican government for financial support under CONACYT-SENER - 2014-01, “Sustentabilidad Energética” with Project No 245750, for funding this work.
References
- Pulz O, Gross W. Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol. 2004; 65: 635–48.
- Lorenz RT, Cysewski GR. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends in Biotechnology. 2000; 18: 160–7.
- León R, Martin M, Vigara J, Vilchez C, Vega JM. Microalgae mediated photoproduction of β-carotene in aqueous–organic two phase systems. Biomolecular Engineering. 2003; 20: 177–82.
- Kim MK, Park JW, Park CS, Kim SJ, Jeune KH, et al. Enhanced production of Scenedesmus spp. (green microalgae) using a new medium containing fermented swine wastewater. Bioresource Technology. 2007; 98: 2220–8.
- Sánchez JF, Fernández JM, Acién FG, Rueda A, Pérez-Parra J, et al. Influence of culture conditions on the productivity and lutein content of the new strain Scenedesmus almeriensis. Process Biochemistry. 2008; 43: 398–405.
- Bhosale P, Bernstein PS. Microbial xanthophylls. Appl Microbiol Biotechnol. 2005; 68: 445–55.
- Ben-Amotz A. Dunaliella β-Carotene. In: Seckbach J, editor. Enigmatic Microorganisms and Life in Extreme Environments. Dordrecht: Springer Netherlands; 1999. 399–410.
- Guerin M, Huntley ME, Olaizola M. Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol. 2003; 21: 210–6.
- Coesel SN, Baumgartner AC, Teles LM, Ramos AA, Henriques NM, et al. Nutrient limitation is the main regulatory factor for carotenoid accumulation and for Psy and Pds steady state transcript levels in Dunaliella salina (Chlorophyta) exposed to high light and salt stress. Mar Biotechnol (NY). 2008; 10: 602–11.
- Mogedas B, Casal C, Forján E, Vílchez C. beta-carotene production enhancement by UV-A radiation in Dunaliella bardawil cultivated in laboratory reactors. J Biosci Bioeng. 2009; 108: 47–51.
- Byreddy AR, Gupta A, Barrow CJ, Puri M. A quick colorimetric method for total lipid quantification in microalgae. Journal of Microbiological Methods. 2016; 125: 28–32.
- Abd El-Baky HH, El-Baroty GS. WITHDRAWN: Enhancement of carotenoids in Dunaliella salina for use as dietary supplements and in the preservation foods. Food Chem Toxicol. 2010.
- Mishra SK, Suh WI, Farooq W, Moon M, Shrivastav A, et al. Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresource Technology. 2014; 155: 330–3.
- Hajare R, Ray A, Shreya, Tharachand C, Avadhani MN, et al. Extraction and quantification of antioxidant lutein from various plant sources. International Journal of Pharmaceutical Sciences Review and Research. 2013; 22: 152–7.
- Zheng H, Gao Z, Yin F, Ji X, Huang H. Lipid production of Chlorella vulgaris from lipid-extracted microalgal biomass residues through two-step enzymatic hydrolysis. Bioresource Technology. 2012; 117: 1–6.
- Vandamme D, Foubert I, Fraeye I, Meesschaert B, Muylaert K. Flocculation of Chlorella vulgaris induced by high pH: role of magnesium and calcium and practical implications. Bioresour Technol. 2012; 105: 114–9.
- Chang YR, Lee DJ. Coagulation–Membrane Filtration of Chlorella vulgaris at Different Growth Phases. Drying Technology. 2012; 30: 1317–22.
- Spilling K, Seppälä J, Tamminen T. Inducing autoflocculation in the diatom Phaeodactylum tricornutum through CO2 regulation. J Appl Phycol. 2011; 23: 959–66.
- Richmond A, editor. Handbook of Microalgal Mass Culture (1986). Boca Raton: CRC Press. 2017; 536.
- Fleurence J. The enzymatic degradation of algal cell walls: a useful approach for improving protein accessibility? Journal of Applied Phycology. 1999; 11: 313–4.
- Molina Grima E, Belarbi EH, Acién Fernández FG, Robles Medina A, Chisti Y. Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv. 2003; 20: 491–515.
- Chisti Y. Biodiesel from microalgae. Biotechnology Advances. 2007; 25: 294–306.
- Mercer P, Armenta RE. Developments in oil extraction from microalgae. European Journal of Lipid Science and Technology. 2011; 113: 539–47.
- Brennan L, Owende P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renewable and Sustainable Energy Reviews. 2010; 14: 557–77.
- Yusaf T, Baker P, Hamawand I, Noor MM. Effect of Compressed Natural Gas Mixing on the Engine Performance and Emissions. International Journal of Automotive and Mechanical Engineering. 2013; 8: 1416–29.
- Mata TM, Martins AA, Sikdar SK, Costa CAV, Caetano NS. Sustainability Considerations about Microalgae for Biodiesel Production. In: Lee JW, editor. Advanced Biofuels and Bioproducts. New York, NY: Springer. 2013; 745–57.
- Ferrer-Alvarez YI, Ortega-Clemente LA, Perez-Legaspi IA, Hernandez-Vergara MP, Robledo-Narvaez PN, et al. Growth of Chlorella vulgaris and Nannochloris oculata in effluents of Tilapia farming for the production of fatty acids with potential in biofuels. African Journal of Biotechnology. 2015; 14: 1710–7.
- Liang Y, Sarkany N, Cui Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett. 2009; 31: 1043–9.
- Bruno B, Ramakrishnan U, Sandhya S. Biomass and Lipid Productivity by Four Fresh Water Microalgae in Photoreactor. Journal of Modern Biotechnology. 2013; 2: 82–8.
- Corrêa PS, Morais Júnior WG, Martins AA, Caetano NS, Mata TM. Microalgae Biomolecules: Extraction, Separation and Purification Methods. Processes. 2021; 9: 10.
- Kadir WNA, Lam MK, Uemura Y, Lim JW, Kiew PL, et al. Simultaneous harvesting and cell disruption of microalgae using ozone bubbles: optimization and characterization study for biodiesel production. Front Chem Sci Eng. 2021; 15: 1257–68.