
Review Article
Ann Agric Crop Sci. 2023; 8(1): 1127.
Emerging Trends on Crosstalk of Jasmonates with Other Phytohormones under Plant Stress
Ghorbel M¹; Brini F²*
1Department of Biology, College of Sciences, University of Hail, Saudi Arabia
2Biotechnology and Plant Improvement Laboratory, Centre of Biotechnology of Sfax, Tunisia
*Corresponding author: Faiçal Brini, Biotechnology and Plant Improvement Laboratory, Centre of Biotechnology of Sfax, B.P ‘1177’, 3018 Sfax, Tunisia. Email: faical.brini@cbs.rnrt.tn
Received: March 13, 2023 Accepted: April 15, 2023 Published: April 22, 2023
Abstract
Plant hormones play crucial and basic roles in plant growth, developmental processes, and also in plant response to abiotic and biotic constraints. On the first time, plant hormones may allocate limited resources to the most serious stresses, on the second time, the crosstalk among multiple plant hormone signaling directs the balance between the plant growth and the plant defense. Various studies and investigations have reported the mechanism of crosstalk between Jasmonic Acid (JA) and other plant hormones in plant growth and stress responses. Based on these investigations, this chapter mainly reports the crosstalk between JA and other plant hormone signaling in regulating the balance between the plant growth and the defense response. The suppressor proteins JASMONATE ZIM DOMAIN PROTEIN (JAZ) and MYC2 as the key components in the crosstalk are also highlighted in the chapter. Eventually, we note that JA interacts with other hormone signaling pathways [such as Ethylene (ET), auxin, Gibberellic acid (GA), Abscisic Acid (ABA), Salicylic Acid (SA) and Brassinosteroids (BRs)] to regulate plant growth, abiotic stress tolerances, and defense resistance against pathogens.
Keywords: Jasmonic acid; Plant hormone; Environmental constraints; Defense response; Crosstalk
Introduction
During development and growth processes, plants are constantly battling against a challenging environment. These adverse environmental conditions are often categorized as: (i) Abiotic constraints, such like nutrient deficiency, Ultraviolet (UV) radiation, flood, drought, heavy metal toxicity and heat, cold and (ii) Biotic constraints, such as pathogen infection and animal herbivory [1]. Under multiple environmental constraints, the phytohormones allocate limited resources to respond to the most serious stress [2] and develop multiples signaling pathways [3,4] to govern the balance between the plant growth and the defense response [2]. Phytohormones are small endogenous signaling molecules, including Gibberellin (GA), Auxin (indole3-acetic acid, IAA), Cytokinin (CK), Brassinosteroids (BRs), Abscisic Acid (ABA), Ethylene (ET), Jasmonic Acid (JA), Salicylic Acid (SA), and Strigolactone (SL). In recent decades, JA anabolism has been widely studied and investigated in monocotyledons and dicotyledons. Indeed, in Arabidopsis, at least two pathways encode JA biosynthesis, namely, the a-linolenic acid (18:3) initial octadecane pathway and hexadecatrienoic acid (16:3) initial hexadecane pathway [5,6]. In those pathways, the 18:3 and 16:3 unsaturated fatty acids are converted to 12-oxo-phytodienoic acid (12-OPDA) and deoxymethylated vegetable dienic acid (dn-OPDA) in the chloroplast, respectively. Then, JA is formed from 12-OPDA and dn-OPDA through multiple β-oxidation in the peroxisome. Finally, different JA structures such as methyl Jasmonate (MeJA), JA–isoleucine (JA–Ile) and 12-hydroxyjasmonic acid (12-OH-JA) are formed from JA in the cytoplasm. Among these JAs, JA–Ile is the biological active form of JA in plants [7]. JA is widely distributed in plants as a natural plant growth regulator [5-8]. The importance of the crosstalk between JA and other phytohormones in regulating plant stress responses has attracted extensive attention [6-9]. In fact, through the crosstalk network, JAs often work in concert with other phytohormones, such as ABA, auxin, CK, ET, GA and SA, to balance between growth and defense-related processes, thereby conferring plants acclimation to the changing environments [10-12]. Studies in recent decades have remarkably expanded our knowledge on the molecular basis underlying JA biosynthesis, transportation, signal transduction and the crosstalk with other signaling pathways. The importance of JA in many developmental processes, including seedling development, lateral root formation, senescence, flower development, sex determination, and the circadian clock has also been elaborately discussed in several reviews [13-15]. In addition, extensive efforts have been made in elucidating the roles of JA in regulating plant responses to abiotic and biotic stress conditions, as well as the importance of the crosstalk between JA and other phytohormones in thoese regulations [11,16-20]. In this chapter, we focus on recent updates on JA anabolism and signal transduction, the crosstalk complexity between JA and other phytohormone signaling during plant development and stress responses, as well as the roles of the involved Transcription Factors (TFs) and other regulatory proteins.
JA Anabolism
To date, three JA anabolic pathways have been detected and identified in Arabidopsis: (1) the octadecane pathway with a-linolenic acid (a-LeA, 18:3) used as precursor, (2) the hexadecane pathway with hexadecatrienoic acid (16:3) used as precursor, and (3) the 12-Oxo-Phytodienoic Acid (OPDA) reductase 3 (OPR3)-independent pathway (Figure 1). All three pathways require multiple enzymatic reactions that take place sequentially in the chloroplast, the peroxisome and finally the cytosol [21]. Concerning the two first pathways, they start with the release of the polyunsaturated fatty acids a-LeA (18:3) and hexadecatrienoic acid (16:3) hydrolyzed from the membrane of chloroplast or plastid depending on the cell type. Through a sequential series of reactions catalyzed by 13-lipoxygenase (13-LOX), Allene Oxide Synthase (AOS) and Allene Oxide Cyclase (AOC), both the 18:3 and 16:3 are converted to OPDA and dnOPDA. Then, OPDA is transported from chloroplast into the peroxisome, where it gets reduced by OPR3 and subsequently shortened by three β-oxidation rounds, finally yielding JA[(+)-7-iso-JA] (Figure 1). dnOPDA is believed to follow the same pathway as OPDA to produce JA with one less β-oxidation round [22]. Upon release into the cytosol, JA is then metabolized into a variety of structures through different reactions, such as conjugation with amino acids, hydroxylation, carboxylation, and methylation, giving birth to a collection of JA derivatives with different biological activities [9,23,24]. Among them, the JA conjugation to the isoleucine by jasmonyl-isoleucine synthetase (JAR1) forms the most bioactive form of the hormone, i.e., (+)-7-iso-JA-Ile (JA-Ile) [25]. When transferred into the cell nucleus, the bioactive JA-Ile, through a “relief of repression” model, activates several key TFs, such as MYC2, for downstream JA-responsive gene expression [26-28]. The OPR3-independent pathway was recently identified by studying a total loss-of function OPR3 mutant, opr3-3 [29]. In the absence of OPR3 activity, OPDA can directly enter the β-oxidation pathway to form dnOPDA, which then gets converted into 4,5-didehydro-JA (4,5-ddh-JA) through two more rounds of β-oxidation. Lastly, 4,5-ddh-JA is reduced to JA by OPR2 in the cytosol (Figure 1). Nevertheless, the majority of JA biosynthesis still occurs through OPR3 [29].
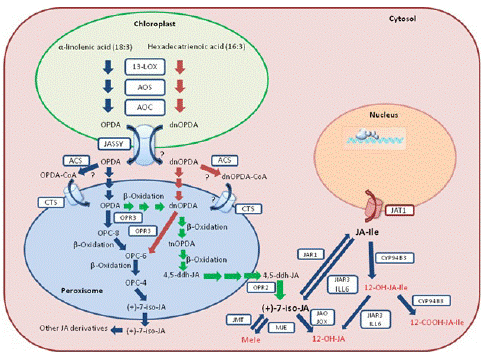
Figure 1: Simplified JA biosynthetic and metabolic pathways and intracellular flux in Arabidopsis. The blue arrows represent the octadecane pathway, the red arrows represent the parallel hexadecane pathway, and the green arrows represent the OPR3-independent pathway. Biologically inactive JA derivatives are shown in red. Biosynthetic and metabolic enzymes, as well as transporters are boxed. 13-LOX: 13-lipoxygenase; AOS: Allene Oxide Synthase; AOC: Allene Oxide Cyclase; OPR: OPDA Reductase; ACS: Acyl-CoA Synthetases; JAR1: JA-Amido Synthetase; IAR3 and ILL6: Two JA Amidohydrolases; JMT: JA Methyl Transferase; MJE: MeJA Esterase; JAO: JA Oxidase; JOX: Jasmonate Induced Oxidase; CYP94B3: JA-Ile-12-Hydroxylase; CYP94C1: 12-OH-JA-Ile Carboxylase; JASSY: OPDA Transporter; CTS: ABC Transporter COMATOSE; JAT: Jasmonate Transporter. dnOPDA: Dinor-Oxo-Phytodienoic Acid; tnOPDA: Tetranor-OPDA; OPC-8: 8-[3-oxo-2-{pent- 2-enyl}cyclopentyl]octanoic Acid; OPC-6: 6-[3-oxo-2-{pent-2-enyl}cyclopentyl]hexanoic Acid; OPC- 4: 4-[3-oxo-2-{pent-2-enyl}cyclopentyl]butanoic Acid; 4,5-ddh-JA: 4,5-didehydro-jasmonate; JA-Ile: (+)-7-iso-Jasmonoyl-L-Isoleucine.
JA Perception and Signaling
JA signal perception and transduction implicate various TFs, repressors, and members of ubiquitin proteasomal pathway. The current JA model signaling transduction is illustrated in Figure 2.
SKP1/CULLIN/F-Box (SCF)COI1 Complex
Exhaustive genetic screens identified the allele of coronatine insensitive1 (coi1), suggesting COI1 functions in JA perception in plants. It was considered as the receptor from two lines of evidence-first, coi1 mutant exhibits male sterility, defective responses to JA-treatment and wounding and susceptibility to necrotrophic pathogens and insects; secondly, COI1 locus encodes an F-box protein that associates with its other counter parts SKP1, Cullin, and Rbx proteins to form an E3 ubiquitin ligase [30,31]. COI1 show approximately 33% sequence similarity with the auxin receptor TIR1 in amino acid sequence having leucine-rich-repeats and F-box motif [32].
JAZ Proteins
After the discovery of the receptor, the most fascinating question was to find out the substrate for SCFCOI1 E3 ubiquitin ligase complex. This substrate was anticipated to be the key negative regulator of JA signaling. In 2007, three independent research groups discovered a new family of protein in Arabidopsis called JASMONATE ZIM DOMAIN (JAZ) proteins [26,33,34]. The JAZ proteins belong to the larger plant specific TIFY family, consisting of a core TIF[F/Y]XG motif within the ZN-FINGER PROTEIN EXPRESSED IN INFLORESCENCE MERISTEM (ZIM) protein domain. A. thaliana consists of 12 JAZ proteins [26,33-35] that are differentiated from other TIFY family proteins by the presence of C-terminally located Jas’s motif, SLX2FX2KRX2RX5PY [34,36,37]. They contain N-terminal domain, a highly conserved C-terminal Jas’s domain that mediates the interaction with the COI1 and several transcription factors, and the conserved protein-protein interaction domain, the ZIM (TIFY) domain that helps in JAZ dimerization and interaction with NINJA and NINJA recruits general transcriptional co-suppressor TPL through the conserved EAR domain [28,35,37,38]. Moreover, it competes with MEDIATOR25 (MED25) to interact with MYCs [39]. The Jas domain is exclusively required to repress downstream targets of JAZ proteins [26,33,34]. The initial clue about the role of JAZ proteins in JA signaling came from the Jasmonate-Insensitive 3 mutant (jai3), which is a mutant of JAI3/JAZ3 gene. In jai3-1 mutant, the JAZ3 protein lacks the C-terminal portion which perturbs its binding and degradation via SCFCOI1 complex. This resulted in accumulation of truncated JAI3/JAZ3 proteins in the mutant which blocked the JA-induced degradation of other JAZ proteins and hence dominant JA-insensitive phenotype [26].
Co-Receptor Complex
The co-receptor complex is formed by the physical interaction of COI1 with the Jas domain of JAZ proteins in the presence of JA-Ile [27,32]. More recently, the role of Inositol Pentakisphosphate (IP5) as a cofactor in the formation of co-receptor complex has been substantiated [27,40]. JA, OPDA, MeJA, and JA-Ile were tested for affinity in COI1JAZ1 binding. Surprisingly, only JA-Ile functioned as ligand for COI1-JAZ interaction [33]. Based on the information available hitherto, the true jasmonates receptor is a co-receptor complex, consisting of the SCFCOI1 E3 ubiquitin ligase complex, JAZ degrons (JAZ1 to JAZ12) and IP5 [27].
Co-repressors
Co-repressors are transcriptional regulators that inhibit transcription initiation. One such example is the group of Groucho/Tup1 corepressor family comprising of TOPLESS (TPL) and TPL-related proteins (TPRs). TPL and TPR mediate repression by recruiting histone deacetylases and demethylases that cause chromatin modification [41]. TPL interacts with JAZ proteins via ETHYLENE RESPONSE FACTOR (ERF)-ASSOCIATED AMPHIPHILIC REPRESSION (EAR) motif. Those JAZ proteins that do not have the repression motif recruit TPL through an adapter protein called NOVEL INTERACTOR OF JAZ (NINJA) [42]. NINJA was first identified by Tandem affinity purification as an interactor of JAZ1 [42].
JAZ Targets
The role of bHLH transcription factor MYC2 in mediating the transcriptional regulation of JA is well defined and thus has been considered the master regulator of many biological processes [43,44]. The role of MYC2 in JA mediated responses is revealed by the study of its mutant Jasmonate-Insensitive1 (jin1). Microarray analysis of wild type and the mutant myc2/jin1 exposed the MYC2 role in JA- dependent transcriptional regulation. MYC2 has twin function of an activator of JA-induced root growth inhibition, anthocyanin biosynthesis and oxidative stress tolerance and a repressor in mediating resistance to necrotrophic pathogens, insects and biosynthesis of tryptophan and indol glucosinolates [43,44]. Besides MYC2, several other TFs control diverse JA response. These TFs are MYC3, MYC4, MYB, GL3, EGL3 AP, GL1 etc. [45]. MYC2 forms homo or heterodimers with its close homologs MYC3 and MYC4 to regulate the transcription of downstream targets [46] by binding to the G-box (5'-CACGTG-3') and G-box related hexamers [47,48]. Moreover, it participates in the crosstalks among JA, ABA, auxin, ET, GA, and other signaling pathways [49].
Roles of SCFCOI1 Complex, MYC2, and JAZ in JA Signaling Pathway
COI1 protein, JAZ, and MYC constitute the core signal transduction mechanism of JA signaling and have been proven to be the intersection of other signal transduction pathways under various stresses (Figure 2). JAZ and various TFs form specific JAZ/TFs that specifically regulate multiple downstream responses [50]. The JAZ-MYC module increases the concentration of defense compounds to trigger defense response or inhibit plant growth against pathogen infection [51]. In addition to the JAZ-MYC module, COI1- JAZ2-MYC2, 3,4-ANAC19,55,72 [52], and other specific JAZ-TF modules have been identified [53,54]. The interaction between MYCs and JAZs may implicate other plant hormone signaling pathways such as ET-mediated cell division through ET RESPONSE FACTOR (ERF) TFs [39,52,55]. Generally, the endogenous level of biologically active JA (JA– Ile) is kept very low plants but can be rapidly activated in response to various stresses such as insect feeding or wounding. Then JA signaling is perceived by JA receptor COI1, an important component of SCFCOI1, which binds to JAZs for ubiquitination and degradation through the 26S proteasome pathway. The competitive binding and degradation of JAZ repressors can further release the downstream TFs such as MYCs, resulting in the activation of JA responses by MYCs [52,55]. To sum up, the three main core components of JA signaling play an important role in plant growth, development, and response to biotic or abiotic stresses. When exogenous JA or extreme stress is applied, the concentration and application time may affect the transcriptional activities of different components in the JA signal module. Therefore, according to the JA metabolic pathway under stress conditions, corresponding stress-tolerance breeding may be developed to improve crop resistance in agriculture.
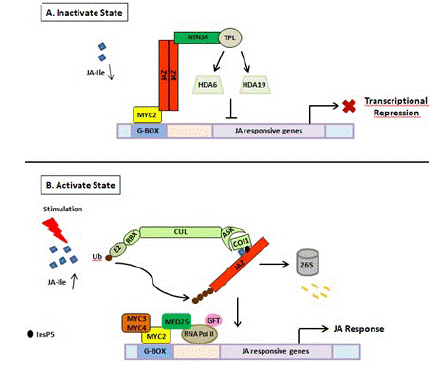
Figure 2: Schematic representation of JA perception and signal transduction pathway. (A) In the absence of stimuli, JA is not synthesized. As a result, JA mediated gene expression is inhibited due to the binding of JAZ repressors to the transcriptional activator MYC2. JAZ proteins recruit TPL and adaptor protein NINJA. Together, JAZ-NINJA-TPL form an active transcriptional repression complex that inhibits jasmonate responses by converting an open complex to a closed complex by recruiting HDA6, HDA19. (B) Upon stimulation by pathogen/insect/wounding, JA is rapidly synthesized and readily epimerizes to JA-Ile. It then binds to COI1-JAZ-InsP5 co-receptor complex causing ubiquitination and proteasomal degradation of JAZ. This frees MYC2 and its homologs from repression which then bind to G-box element present downstream of JA-responsive genes upon homo/heterodimerization. This is followed by the recruitment of MED25 that in turn bring RNAPol II and general transcription factors hence, causing diverse jasmonate responses. JA: Jasmonic Acid; JA-Ile: Jasmonate–isoleucine; JAZ: Jasmonate ZIM Domain; NINJA: Novel Adaptor of JAZ; TPL: Topless; COI1: Coronatine Insensitive; Cul: Cullin1; RBX1: Ring Box1; Ub: Ubiquitin; ASK1: Arabidopsis Skp1 Homolog1; InsP5: Inositol Pentakisphosphate; GTF: General Transcription Factor; HDA6, HDA19: Histone Deacetylase 6, 19; MED25: Mediator25; RNAPol II: RNA Polymerase II.
JA Regulates Plant Response to Biotic/Abiotic Stresses
Plants encounter numerous challenges in terms of competition from other plants, organisms and because of the complex environment. All these provocations have made the plants tougher and more flexible. The morphological flexibility has given them the advantage to counteract, inhabit and endure biotic and abiotic challenges. Rapid changes in the plant biochemistry and physiology are mediated by the action of several phytohormones. By tradition CK, auxins, BR, and GA have always been associated to regulate developmental processes of plants, whereas SA, JA and ET associate with plant defense and ABA regulates plant’s response to abiotic stress. Now, it has been quite evident from many reports that all hormones affect multiple plant functions. Thus, one can say that hormones not only participate in plant developmental processes but also have a say in plant’s response to abiotic stresses like drought, osmotic stress, chilling injury, heavy metal toxicity etc. These adversities have forced the plants to either employ avoidance as a mechanism in order to surmount the stress or choose defense overgrowth [38,56-58]. Thus, stress activates signal transduction of hormones which may promote specific protective mechanisms.
JA and Cold Stress
In order to adapt to extremely low temperatures, plants have evolved complex mechanisms by regulating physiological and biochemical processes, especially the modulation of stress-related gene expression. The INDUCER OF CBF EXPRESSION (ICE)- CBF transcriptional cascade signaling pathway plays a central role in plant cold stress response [59]. ICE1 and ICE2, two Basic Helix–Loop–Helix (bHLH) TFs in A. thaliana, upregulate the expressions of CBFs through directly binding to CANNTG in the promoter region of CBFs.
In Rice growing at normal temperature, JAZ1 and JAZ4 interact with ICE1 and ICE2 to inhibit the ICE-CBF signaling pathway (Figure 3). Under low-temperature conditions, the expressions of JA synthesis-related genes including allene oxide synthase1 (AOS1), DAD1, Allene Oxide Cyclase (AOC), LOX2, and AOS1 are induced [1,59], and bioactive JA–Ile is synthesized, thereby activating JA receptor COI1 to bind to JAZ1, resulting in the degradation of JAZ1 through 26S proteome after ubiquitination (Figure 3). Then, the ICE-CBF transcriptional regulation cascade signaling pathway is activated, and the expressions of cold-regulated genes are induced to improve plant cold tolerance.
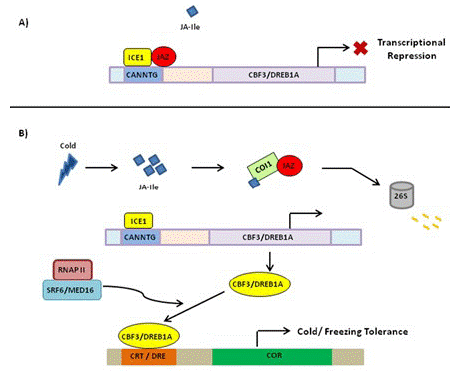
Figure 3: Schematic representation of regulation of cold stress tolerance by JA signal transduction pathway. (A) Under normal growth conditions, JAZ repressor proteins physically interact and suppress cold TF ICE1, thus repressing ICE/CBF-DREB1 pathway and rendering plants sensitive to freezing. (B) Upon cold induction, JA is synthesized that rapidly isomerizes to JA-Ile and lead to proteasomal degradation of JAZ. This frees ICE1 that binds to CBF3 responsive element leading to its expression. The CBF proteins bind to CRT/DRE element causing the expression of COR genes that participate in cold/freezing tolerance. JAZ: Jasmonate ZIM Domain Protein; TF: Transcription Factor; ICE1: Inducer of CBF Expression; CBF-DREB1: C Repeat Binding Factor1-Dehydartion Responsive Element Binding Factor 1B; COR: Cold Regulated; DRE: Dehydration Responsive Element; CRT: C-Repeat; SFR6/MED16: Sensitive to Freezing6/Mediator16; RNAP II: RNA Polymerase II; COI1: Coronatine Insensitive.
JA and Drought Stress
Drought stress response is a complex process in plants. Stomatal closure can reduce water loss and is a potential drought resistance mechanism of plants [60]. JA and JA precursor 12-OPDA can promote stomatal closure in A. thaliana, and the increase of OPDA content is related to the decrease of stomatal aperture and improved drought resistance [61]. 13-Lipoxygenase LOX6 is essential for the synthesis of 12-OPDA and plays an important role in plant drought tolerance. In rice, when drought stress is applied, LOX6 is synthesized to 12-OPDA, which promotes stomatal closure and improves drought tolerance in the presence or absence of ABA (Figure 4). In response to drought stress, the JA signaling pathway is activated, and OsbHLH148 interacts with OsJAZ1 to activate the expression of OsDREB1, thereby improving drought tolerance in rice (Figure 4) [62]. Moreover, some antioxidant enzymes, including Superoxide Dismutase (SOD), Peroxidase (POD), Catalase (CAT), proline, and Relative Water Content (RWC), are increased to enhance the ability of plants to cope with drought stress [62].
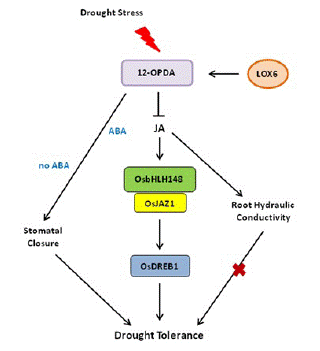
Figure 4: JA-mediated drought stress response in plants. In response to drought stress, the JA signaling pathway is activated; OsbHLH148 interacts with OsJAZ1 to activate the expression of OsDREB1, together with JA-mediated root hydraulic conductivity and stomatal closure, thereby improving drought tolerance in rice.
JA and Heavy Metal/Metalloid
Although many metal ions are essential nutrients some are toxic to both plants and animals [63,64]. Excess of essential metals or even metalloid induce toxicity in plants, which may result into oxidative stress leading to physiological changes [65]. JA application, however, enhanced accumulation of osmolytes while carotenoids enhanced antioxidant enzyme concentration which prevented the plants from damage by excess metal ions [66]. Excess cadmium produces ROS, H2O2, and superoxide radicals which cause oxidative damage in plants [67]. However, MJ application reduces this damage by about 30% in soybean and A. thaliana [68]. Excess boron in the soil may be absorbed by plants and can cause visible damage such as leaf burn, decreased fruit size [69] besides ROS production in wheat [70], barley [71], and tomato [72]. Foliar spray of MJ alleviated the above symptoms significantly by stimulating the antioxidant production with a consequent reduction in lipid peroxidation. Heavy metal stress is also alleviated by activating the antioxidant system [73]. MJ strengthened tolerance in A. thaliana plants against copper and cadmium stress through accumulation of chelating ligands which form complex with metal ions and prevent their availability to plant. Singh and Shah [74] reported that under cadmium stressed O. sativa, application of MJ exhibited remarkable changes in activity of CAT, SOD, and GR paralleled with increased glutathione pools.
JA and Fungal diseases
Hemibiotrophic and necrotrophic fungi have a wide host range, resulting to serious yield losses in many important crops [75,76]. JA plays an important role in inducing plant against necrotrophic and hemibiotrophic pathogen and herbivorous insect feeding [76-78]. Some hemibiotrophic fungi can metabolize JA produced by host plants. The Antibiotic Biosynthetic Monooxygenase (Abm) formed by Magnaporthe grisea can convert JA into 12-OH-JA to weaken JA signaling and promote the colonization of Magnaporthe oryzae [79,80]. When rice blast fungus and rice are incompatible, Abm secreted by rice blast fungus is degraded, resulting in the accumulation of MeJA and the activation of JA downstream response as well as immuneresponse (Figure 5A). So far, the JA–MYC2–PDF1.2 module is widely involved in plant– fungi interaction [80]. With the effect on promoting pathogenesis, the expression of LOB DOMAIN-CONTAINING PROTEIN 20 (LBD20) is closely related to VSP2 and THIONIN 2.1 (Thi2.1) as well as MYC2 [81]. MED19a is an important member of the mediator co-activator complex in JA signaling, and it can be degraded by an oomycete effector protein HaRxL44 [82]. We have to note that the underlying mechanism of the JA signaling pathway in plant–fungi interaction remains elusive and needs to be further investigated.
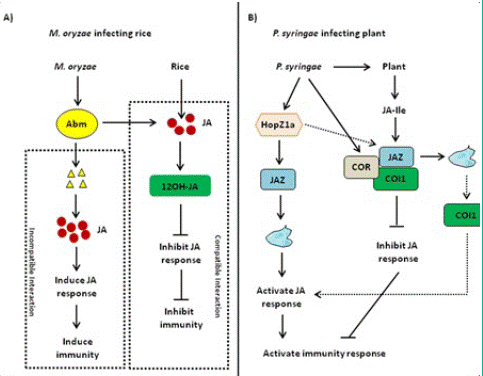
Figure 5: Jasmonic Acid (JA)-mediated disease resistance against Magnaporthe oryzae and Pseudomonas syringae. (A) Jasmonic Acid (JA)-mediated disease resistance against Magnaporthe oryzae in rice. When rice blast fungus is compatible with rice, rice blast fungus secretes Antibiotic Biosynthetic Monooxygenase (Abm) and inhibits JA activity and immune response. When rice blast fungus and rice are incompatible, Abm secreted by rice blast fungus is degraded, resulting in the accumulation of Methyl Jasmonate (MeJA) and the activation of JA downstream response as well as immune response. (B) JA-mediated disease resistance against Pseudomonas syringae in Arabidopsis. HopZ1a directly interacts with JASMONATE ZIM DOMAIN PROTEIN (JAZ) proteins and induces the acetylation of JAZ proteins, thereby activating the JA signaling pathway. As one kind of functional JA analog, Coronatine (COR) can induce CORONATINE INSENSITIVE 1 (COI1) to bind to JAZ proteins, thereby activating JA downstream response and plant immune response.
JA and Bacterial Diseases
Many pathogenic variants of hemibiotrophic bacteria Pseudomonas syringae can produce polyketide toxin coronatine (COR), AvrB, and HopZ1a. The most widely known example of JA-mediated plant–pathogen interaction is regulated by COR. COR is an active substance similar to JA–Ile in structure and function, with two moieties including coronamic acid and coronafacic acid [83]. On the one hand, COR can promote bacterial infection through the modulation of pathogen-associated molecular Pattern-Triggered Immunity (PTI)-activated stomatal closure and defense response [27,31]. On the other hand, COR can directly bind to the COI1–JAZ complex, and the activation of COR-mediated JA signaling pathway inhibits SA-mediated plant defense resistance against P. syringae infection [31,84]. In addition, COR has a toxic function by regulating secondary metabolites and inhibiting callose formation, which is independent of plant hormone antagonism [85-87]. Therefore, JA–Ile mimics such as COR may be essential for the infection of some bacterial pathogens. COR can also enhance the interaction between COI1 and JAZ proteins [31,88,89]. AvrB regulates JA signaling through modulation of the COI1- dependent manner in Arabidopsis [90]. In this case, the Arabidopsis protein RPM1-INTERACTING PROTEIN 4 (RIN4) appears to be involved [88,91]. AvrB interacts with RIN4 and triggers the plasma membrane localized AHA1. Both AvrB and AHA1 promote the interaction between COI1 and JAZ, thereby regulating stomatal opening and plant defense response [88]. Unlike COR and AvrB, as an acetyl transferase, HopZ1a directly interacts with JAZ proteins and induces the acetylation of JAZ proteins, thereby activating the JA signaling pathway (Figure 5B) [92].
Crosstalk between JA and Other Plant Hormone Signaling Pathways
The crosstalk between plant hormones is the core of plant stress response [90]. JA does not work independently but acts in a complex signaling network combined with other plant hormone signaling pathways [6-8,59]. As a core component of JA signaling, the role of JAZs-MYC2 in the crosstalk of plant hormone signaling pathways is highlighted in this chapter, especially in the crosstalk of JA–auxin, JA–GA, JA– ABA, JA–ET, JA–SA, JA–BR, and signaling pathways [20,50,93-95] (Figure 6).
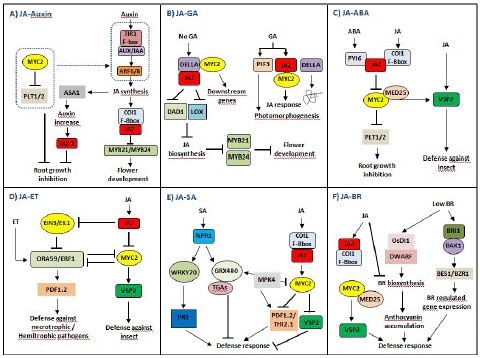
Figure 6: JASMONATE ZIM DOMAIN PROTEIN (JAZ)-mediated crosstalks among JA hormone signaling pathways in plant growth and stress responses. (A) The complex crosstalk between JA and auxin signaling pathways. JA and auxin signaling pathways coordinately regulate flower development through modulation of JA, while JA and auxin antagonize root growth through JAZs-MYC2. (B) The complex crosstalk between JA and GA signaling pathways. The JAZ-MYC2-DELLA-PIF signaling module being involved in the crosstalk between JA and GA signaling can be elucidated. In addition, many Transcription Factors (TFs) such as MYC3, MYC4, MYB21, and MYB24 can also interact with DELLAs, so there may be synergistic effect between JA and GA signaling. (C) The complex crosstalk between JA and Abscisic Acid (ABA) signaling pathways. The crosstalk between PYRABACTIN RESISTANCE1-Like protein (PYL) and JAZ–MYC2 coordinates the balance between plant growth and defense resistance. (D) The complex crosstalk between JA and Ethylene (ET) signaling pathways. JA and ET coordinately regulate plant stress responses through JAZs-MYC2 and EIN3/EIL1, especially in resisting necrotrophic or hemibiotrophic pathogens. (E) The complex crosstalk between JA and SA signaling pathways. SA initiates early defense-related gene expression in pathogen-infected plants, while JA induces late defense-related gene expression in pathogen-infected plants, mainly in the necrotrophic stage of necrotrophic or hemibiotrophic pathogens. (F) The complex crosstalk between JA and Brassinosteroids (BR) signaling pathways. The crosstalk between JA and BR biosynthesis may be involved in the balance between plant growth and defense resistance.
JA-Auxin Crosstalk
JA and auxin signaling pathways coordinately regulate plant growth and development. COI1, MYC2, and JAZ, as the main core components, participate in the crosstalk of JA and auxin signaling pathways (Figure 6A). When plants are induced by exogenous auxin, the auxin–TIR–AUX/IAA–ARF signaling is activated, and JA synthesis is induced. On the one hand, the endogenous JA induces the expression of auxin synthase gene (ASA1) and auxin content, so JA regulates the biosynthesis of auxin and further regulates the expression of JAZ1 and root growth. On the other hand, JA induces the formation of a complex of COI1 and JAZ and leads to the degradation of JAZ, thereby activating the transcriptional activities of MYB21/MYB24 and inducing flower development. Notably, MYC2 inhibits the expressions of PLETHORAs (PTL1 and PTL2) and counteracts the auxin–TIR–AUX/IAA–ARF signaling, to regulate root growth [96]. In addition, ARF6/ARF8 in the auxin signaling pathway regulates petal and stamen growth through modulation of endogenous JA level, and MYB21/MYB24 as downstream of JAZ in JA signaling pathways also coordinately regulate petal and stamen growth [97]. Thus, JA and auxin signaling pathways coordinately regulate flower development through modulation of JA, while JA and auxin antagonize root growth through JAZs-MYC2.
JA-GA Crosstalk
JA and GA signaling pathways coordinately and antagonistically regulate plant growth and defense response; however, plant defense response is exerted at the cost of inhibiting growth [38,98]. The C-terminus of JAZs is necessary for the interaction between JAZs and MYC2 and between JAZs and DELLAs, so DELLAs can completely interact with JAZs [99]. In the absence of GA, stable DELLA interacts with JAZ to release MYC2, resulting in the activation of MYC2 downstream genes. At the same time, DELLA interacts with JAZ to inhibit the expression of JA biosynthetic genes (DAD1 and LOX) and further inhibits JA biosynthesis as well as the activities of MYB21 and MYB24 (Figure 6B), thereby regulating stamen development [100]. GA induces the degradation of DELLA and the binding of JAZ to MYC2, to inhibit JA signaling. In addition, GA induces PIF3/PIF4 to regulate photomorphogenesis (Figure 6B) [101]. Notably, JA delays GA-mediated degradation of DELLA, the della mutant is less sensitive to JA-inhibited plant growth inhibition, and AtJAZ9 inhibits the interaction between DELLA and PIF3 [98]. Therefore, the molecular cascade involving the JAZMYC2-DELLA-PIF signaling module in the crosstalk between JA and GA signaling pathways can be elucidated. Moreover, many TFs such as MYC3, MYC4, MYB21, and MYB24 can also interact with DELLAs, so there may be a synergistic effect between JA and GA signaling pathways [102].
JA-ABA Crosstalk
ABA and JA signaling pathways coordinately regulate plant response to herbivorous insect feeding while antagonizing plant growth and development. JAZs-MYC2 participates in the crosstalk between JA and ABA signaling pathways, affecting plant growth and defense [96]. ABA receptor PYRABACTIN RESISTANCE1-Like proteins (PYLs) regulate metabolic reprogramming in Arabidopsis thaliana and tobacco through the JA signaling pathway. Therefore, the crosstalk between ABA and JA signaling pathways can monitor elicitor induced reprogramming of plant metabolism and growth [103]. ABA receptor PYL forms a complex with JAZ, which activates the transcriptional activated activity of MYC2. On the one hand, MYC2 activates the expression of JA responsive gene VSP2 under the mediation of MED25 to resist herbivorous insect feeding. On the other hand, MYC2 inhibits the expressions of PTL1 and PTL2 as well as root growth. Additionally, ABA initiates the degradation of JAZ12, which plays a specific role in the crosstalk between JA and ABA signaling pathways [104]. Thus, the crosstalk between JA and ABA signaling, especially between PYL and JAZ-MYC2, coordinates the balance between plant growth and defense resistance (Figure 6C).
JA-ET Crosstalk
JA and ET antagonize or coordinately regulate plant stress response [105,106] (Figure 6D). ET INSENSITIVE3 (EIN3) and its homologue EIN3-like 1 (EIL1) in the ET signaling pathway as well as JAZs-MYC2 in the JA signaling pathway are involved in the crosstalk between JA and ET signaling pathways [107]. On the one hand, exogenous JA triggers the degradation of JAZ, and the release of MYC2 regulates the expression of ORA59/ERF1 and wound responsive gene VSP2, to resist herbivorous insects.
On the other hand, JAZ inhibits the transcriptional activity of EIL2/EIN3 in the ET signaling pathway and activates downstream ORA59/ERF1 that targets the promoter of PLANT DEFENSIN 1.2 (PDF1.2) and induces its expression, thereby resisting the infection of necrotrophic pathogens and hemibiotrophic pathogens [108]. Generally, the JA signaling pathway synergistically crosstalk with the ET signaling pathway against necrotrophic pathogen attacks and activates the expression of defense proteins such as PDF1.2 through ERF1 and ORA59. Thus, JA and ET coordinately regulate plant stress responses through JAZs-MYC2 and EIN3/EIL1, especially in resisting necrotrophic or hemibiotrophic pathogens [3].
JA-SA Crosstalk
Generally, JA is widely involved in regulating disease resistance against necrotrophic pathogens, while SA mediates broad spectrum resistance against biotrophic and hemibiotrophic pathogens [109]. It has been shown that JA signaling can inhibit SA accumulation through modulation of multiple NAC TFs, such as ANAC019/055/072. Briefly, MYC2 directly binds to the promoters of these NACs and then activates their transcription. Then the activation of these NAC TFs further inhibits the expression of ISOCHORISMATE SYNTHASE 1 (ICS1) as an SA biosynthesis gene while triggering the expression of BENZOIC ACID/SA CARBOXYL METHYLTRANSFERASE 1 (BSMT1) as an SA methylation gene [89]. In addition, the crosstalk between JA and SA signaling pathways involves many components, including redox regulators Glutathione (GRX) and Thioredoxin (TRX) [110], MYC2, TGAs, and PDF 1.2 [111] and WRKY70 [112].
In the presence of exogenous SA, NONEXPRESSOR OF PR GENES1 (NPR1) is activated to induce the transcriptional-activated activity of WRKY70, which promotes the expression of PR1 by binding to the promoter region of PR1 and inducing defense response. In the meanwhile, NPR1 polymers are monomerized by TRX through SA-induced redox state changes, and then monomers such as GRX480 are transported to the nucleus and specifically bind to TGAs, which also directly regulate the expression of PR1 [109,111,113-115] (Figure 6E). Thus, the transformation between NPR1 polymer and monomer has a dual role in inhibiting and activating defense-related gene expression [38]. Interestingly, the induction of GRXs can block TGA-mediated JA response gene expression, such as ORA59, further confirming SA–JA antagonism [115]. MPK4 positively regulates GRX480 in the SA signaling pathway and negatively regulates MYC2 in the JA signaling pathway, which is necessary for JA responsive genes (PDF1.2 and THI2.1) [38]. Therefore, MYC2 and its upstream MPK4 are involved in the crosstalk between JA and SA signaling pathways, which coordinately regulate plant disease resistance against necrotrophic or hemibiotrophic pathogens. SA initiates early defense-related gene expression in infected plants, while JA induces late defense-related gene expression in infected plants, mainly in the necrotrophic stage of necrotrophic or hemibiotrophic pathogens.
JA-BR Crosstalk
JA inhibits plant growth, while BR induces above-ground plant growth. The crosstalk between JA and BR signaling pathways is involved in the balance between plant growth and defense resistance. On the one hand, low concentration of BR induces the expression of OsDI1 and OsDWARF at the early and late stages of BR biosynthesis, respectively, and anthocyanin accumulation and activates defense response.
On the other hand, high concentration of BR activates BR signaling cascades including BR receptor BRI1, BR-related kinase BAK1, and BR-related TFs to induce the expressions of downstream genes such as BES1 and BZR1, thereby regulating plant responses to abiotic stresses (Figure 6F). JA induces JAZ to bind COI1, and MYC2 activates the expression of VSP2 under the mediation of MED2, thereby resisting herbivorous insect feeding (Figure 6F). Notably, high concentration of BR inhibits endogenous biosynthesis of JA and BR, and JA also inhibits BR biosynthesis [116]. Thus, the crosstalk between JA and BR biosynthesis may be involved in the balance between plant growth and defense resistance.
Role of Crosstalk between JA and Other Plant Hormones in Plant Growth and Defense Balance
The crosstalk between plant hormone signaling pathways promote the balance between plant growth and defense [117]. In order to survive and reproduce, plants should not only maintain growth but also resist pathogen infection. Therefore, the balance between plant growth and defense resistance has important ecological, agricultural, and economic values. The JAZ-MYC module in the JA signaling pathway plays a central role in the balance by integrating TF complexes and plant metabolic pathways [118]. When plants are infected by pathogens, PTI as the first defense system of plants, is activated rapidly, followed by SA, JA, and other plant hormone signaling pathways. In the meanwhile, auxin, BR, and GA signaling pathways related to plant growth are inhibited [119,120]. The changes in the amount and composition of stress-related hormones promote plant defense response [121,122]. When plants are subjected to biotic stress, the transient PTI response and the subsequent SA, JA, GA, BR, and other plant hormone signaling pathways have certain persistence [3]. SA signaling is mainly involved in disease resistance against biotrophic pathogens, while JA signaling is mainly involved in disease resistance against necrotrophic pathogens or the necrotrophic stage of hemibiotrophic pathogens [123]. The crosstalk between GA and JA signaling pathways plays a major role in balancing plant growth and defense against biotic and abiotic stresses [98,99,101,124-126]. GA regulates many aspects of plant growth, and JA plays a major role in stress response. JA and BR coordinately regulate plant environmental stresses, while JA and BR antagonize plant growth [127]. BR negatively regulates PTI response, because the inhibition of PTI-induced gene expression may lead to the decrease of BR biosynthesis [128]. In summary, the balance between plant growth and defense disease depends on the crosstalk between PTI, JA, SA, BR, and GA signaling pathways.
Conclusion and Prospect
In nature, plants are often subjected to diver’s abiotic and biotic constraints. In response to those lasts, plants initiate a series of defense responses, PTI and Effector-Triggered Immunity (ETI); among them are the main defense ones. The phytohormone signaling network also plays basic and crucial role in the early regulation of plant defense response as well as plant–pathogen interaction. Because phytohormones are natural and nontoxic in plants and the crosstalk among them regulate the balance between plant growth and defense resistance, it may have a broad application prospect to develop phytohormones as safe and environment-friendly elicitors by utilizing the crosstalk between plant hormone signaling pathways. In recent decades, although the JA signaling pathway has been extensively investigated, the current understanding of its role in different environmental stresses is limited, due to the complex networks and crosstalk between multiple stresses and multiple signaling pathways. So far, the molecular mechanism of JA signaling in stress responses remains elusive. Compared with unidentified components, the identified components in plant hormone signaling pathways are limited. In addition, so many receptors and kinases exist in the cell membrane, and different environmental stresses may activate multiple enzymes, with a series of activation of secondary messengers such as Ca2+ and the reaction of kinase-TF-downstream genes. At present, there are still a lot of questions or gaps in understanding the crosstalk between JA and other hormones in plant stress responses, especially in the perception of multiple environmental signals. With the development of protein interaction omics, the complex protein interaction network may provide more clues to the understanding of complex stress signaling perception and protein complex mediated plant hormone crosstalk. Moreover, data in the lab maybe largely different from those in the field, providing limited information for agriculture production. Therefore, it is necessary to comprehensively analyze plant hormone signaling networks during the whole developmental stages in the field; this will provide more values for crop breeding in the future. Further investigation will provide a novel insight into developing plant hormones for agricultural production by improving stress resistance and crop quality.
References
- Leonardi M, Ustun TB. The global burden of epilepsy. Epilepsia. 2002; 43: 21-25.
- Burneo JG, Jette N, Theodore W, Begley C, Parko K, et al. Disparities in epilepsy: report of a systematic review by the North American Commission of the International League Against Epilepsy. Epilepsia. 2009; 50:2285-2295.
- Angalakuditi M, Angalakuditi N. A comprehensive review of the literature on epilepsy in selected countries in emerging markets. Neuropsychiatric disease and treatment. 2011; 7: 585-597.
- Theodore WH, Spencer SS, Wiebe S, Langfitt JT, Ali A, et al. Epilepsy in North America: a report prepared under the auspices of the global campaign against epilepsy, the International Bureau for Epilepsy, the International League Against Epilepsy, and the World Health Organization. Epilepsia. 2006; 47: 1700-1722.
- Dua T, de Boer HM, Prilipko LL, Saxena S. Epilepsy Care in the World: results of an ILAE/IBE/WHO Global Campaign Against Epilepsy survey. Epilepsia. 2006; 47: 1225-1231.
- Meinardi H, Scott RA, Reis R, Sander JW. The treatment gap in epilepsy: the current situation and ways forward. Epilepsia. 2001; 42: 136-149.
- Connock M, Frew E, Evans BW, Bryan S, Cummins C, et al. The clinical effectiveness and cost-effectiveness of newer drugs for children with epilepsy. A systematic review. Health Technol Assess. 2006; 10: ix-118.
- Wilby J, Kainth A, Hawkins N, Epstein D, Mclntosh H, et al. Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation. Health Technol Assess. 2005; 9: 1-157.
- Schmidt D. Efficacy of new antiepileptic drugs. Epilepsy currents/American Epilepsy Society. 2011; 11: 9-11.
- Glauser T, Ben-Menachem E, Bourgeois B, Cnaan A, Chadwick D, et al. ILAE treatment guidelines: evidence-based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2006; 47: 1094-1120.
- Tolman JA, Faulkner MA. Treatment options for refractory and difficult to treat seizures: focus on vigabatrin. Therapeutics and clinical risk management. 2011; 7: 367-375.
- Morrow J, Russell A, Guthrie E, Parsons L, Robertson I, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. Journal of neurology, neurosurgery, and psychiatry. 2006; 77: 193-198.
- Taylor DC. One hundred years of epilepsy surgery: Sir Victor Horsley’s contribution. Journal of neurology, neurosurgery, and psychiatry. 1986; 49: 485-488.
- Duguid C. Macewen of Glasgow; a recollection of the chief. Edinburgh,: Oliver and Boyd; 1957.
- Schramm J, Clusmann H. The surgery of epilepsy. Neurosurgery. 2008; 62: 463-481.
- Wilson SJ, Engel J Jr. Diverse perspectives on developments in epilepsy surgery. Seizure: the journal of the British Epilepsy Association. 2010; 19: 659-668.
- Jobst BC, Darcey TM, Thadani VM, Roberts DW. Brain stimulation for the treatment of epilepsy. Epilepsia. 2010; 51: 88-92.
- Saillet S, Langlois M, Feddersen B, Minotti L, Vercueil L, et al. Manipulating the epileptic brain using stimulation: a review of experimental and clinical studies. Epileptic disorders: international epilepsy journal with videotape. 2009; 11: 100-112.
- Fisher R, Salanova V, Witt T, Worth R, Henry T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010; 51: 899-908.
- Morrell M. Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures? Current opinion in neurology. 2006; 19: 164-168.
- Theodore WH, Fisher R. Brain stimulation for epilepsy. Acta neurochirurgica. Supplement. 2007; 97: 261-272.
- McClelland S 3rd, Guo H, Okuyemi KS. Population-based analysis of morbidity and mortality following surgery for intractable temporal lobe epilepsy in the United States. Archives of neurology. 2011; 68: 725-729.
- Locharernkul C, Kanchanatawan B, Bunyaratavej K, Srikijvilaikul T, Deesudchit T, et al. Quality of life after successful epilepsy surgery: evaluation by occupational achievement and income acquisition. Journal of the Medical Association of Thailand. 2005; 88: S207-213.
- Tellez-Zenteno JF, Dhar R, Hernandez-Ronquillo L, Wiebe S. Long-term outcomes in epilepsy surgery: antiepileptic drugs, mortality, cognitive and psychosocial aspects. Brain: a journal of neurology. 2007; 130: 334-345.
- Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain: a journal of neurology. 2005; 128: 1188-1198.
- Tellez-Zenteno JF, Hernandez Ronquillo L, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy research. 2010; 89: 310-318.
- Hamiwka L, Macrodimitris S, Tellez-Zenteno JF, Metcalfe A, Wiebe S, et al. Social outcomes after temporal or extratemporal epilepsy surgery: a systematic review. Epilepsia. 2011; 52: 870-879.
- Benifla M, Rutka JT, Otsubo H, Lamberti-Pasculli M, Elliott I, et al. Long-term seizure and social outcomes following temporal lobe surgery for intractable epilepsy during childhood. Epilepsy research. 2008; 82: 133-138.
- Hynes CA, Mar RA. A case study of long-term cognitive and social functioning following a right temporal lobectomy in infancy. Neurocase. 2008; 15: 37-46.
- Langfitt JT, Wood BL, Brand KL, Brand J, Erba G. Family interactions as targets for intervention to improve social adjustment after epilepsy surgery. Epilepsia. 1999; 40: 735-744.
- Derry PA, Rose KJ, McLachlan RS. Moderators of the effect of preoperative emotional adjustment on postoperative depression after surgery for temporal lobe epilepsy. Epilepsia. 2000; 41: 177-185.
- Velasco AL, Boleaga B, Brito F, Jimenez F, Gordillo JL, et al. Absolute and relative predictor values of some non-invasive and in vasive studies for the outcome of anterior temporal lobectomy. Archives of medical research. 2000; 31: 62-74.
- Gonnaud PM. Specific medico-social supports for drug-resistant partial epilepsies]. Revue neurologique. 2004; 160: 5S301-307.
- Wilson SJ, Bladin PF, Saling MM, Pattison PE. Characterizing psychosocial outcome trajectories following seizure surgery. Epilepsy & behavior: E&B. 2005; 6: 570-580.
- Burneo JG, Black L, Martin R, Devinsky O, Pacia S, et al. Race/ethnicity, sex, and socioeconomic status as predictors of outcome after surgery for temporal lobe epilepsy. Archives of neurology. 2006; 63: 1106-1110.
- Dupont S, Tanguy ML, Clemenceau S, Adam C, Hazemann P, Baulac M. Long-term prognosis and psychosocial outcomes after surgery for MTLE. Epilepsia. 2006; 47: 2115-2124.
- von Lehe M, Lutz M, Kral T, Schramm J, Elger CE, et al. Correlation of health-related quality of life after surgery for mesial temporal lobe epilepsy with two seizure outcome scales. Epilepsy & behavior: E&B. 2006; 9: 73-82.
- Bergen DC. Results of epilepsy surgery: still so much to learn. Epilepsy currents/American Epilepsy Society. 2006; 6: 80-82.
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. The New England journal of medicine. 2001; 345: 311-318.
- Elsharkawy AE, Alabbasi AH, Pannek H, Schulz R, Hoppe M, et al. Outcome of frontal lobe epilepsy surgery in adults. Epilepsy research. 2008; 81: 97-106.
- Tonini C, Beghi E, Berg AT, Bogliun G, Giordano L, et al. Predictors of epilepsy surgery outcome: a meta-analysis. Epilepsy research. 2004; 62: 75-87.
- Ryvlin P, Bouvard S, Le Bars D, De Lamerie G, Gregoire MC, et al. Clinical utility of flumazenil-PET versus [18F]fluorodeoxyglucose-PET and MRI in refractory partial epilepsy. A prospective study in 100 patients. Brain: a journal of neurology. 1998; 121: 2067-2081.
- Richardson MP, Koepp MJ, Brooks DJ, Duncan JS. 11C-flumazenil PET in neocortical epilepsy. Neurology. 1998; 51: 485-492.
- Bouvard S, Costes N, Bonnefoi F, Lavenne F, Mauguiere F, et al. Seizure-related short-term plasticity of benzodiazepine receptors in partial epilepsy: a [11C]flumazenil-PET study. Brain: a journal of neurology. 2005; 128: 1330-1343.
- Hammers A, Koepp MJ, Labbe C, Brooks DJ, Thom M, et al. Neocortical abnormalities of [11C]-flumazenil PET in mesial temporal lobe epilepsy. Neurology. 2001; 56: 897-906.
- Koepp MJ, Hammers A, Labbe C, Woermann FG, Brooks DJ, et al. 11C-flumazenil PET in patients with refractory temporal lobe epilepsy and normal MRI. Neurology. 2000; 54: 332-339.
- Ryvlin P, Bouvard S, Le Bars D, Mauguiere F. Transient and falsely lateralizing flumazenil-PET asymmetries in temporal lobe epilepsy. Neurology. 1999; 53: 1882-1885.
- Juhasz C, Nagy F, Muzik O, Watson C, Shah J, et al. [11C]Flumazenil PET in patients with epilepsy with dual pathology. Epilepsia. 1999; 40: 566-574.
- Koepp MJ, Labbe C, Richardson MP, Brooks DJ, Van Paesschen W, et al. Regional hippocampal [11C]flumazenil PET in temporal lobe epilepsy with unilateral and bilateral hippocampal sclerosis. Brain: a journal of neurology. 1997; 120: 1865-1876.
- Koepp MJ, Richardson MP, Labbe C, Brooks DJ, Cunningham VJ, et al. 11C-flumazenil PET, volumetric MRI, and quantitative pathology in mesial temporal lobe epilepsy. Neurology. 1997; 49: 764-773.
- Debets RM, Sadzot B, van Isselt JW, Brekelmans GJ, Meiners LC, et al. Is 11C-flumazenil PET superior to 18FDG PET and 123I-iomazenil SPECT in presurgical evaluation of temporal lobe epilepsy? Journal of neurology, neurosurgery, and psychiatry. 1997; 62: 141-150.
- Richardson MP, Koepp MJ, Brooks DJ, Fish DR, Duncan JS. Benzodiazepine receptors in focal epilepsy with cortical dysgenesis: an 11C-flumazenil PET study. Annals of neurology. 1996; 40: 188-198.
- Chugani HT, Kumar A, Kupsky W, Asano E, Sood S, et al. Clinical and histopathologic correlates of 11C-alpha-methyl-L-tryptophan (AMT) PET abnormalities in children with intractable epilepsy. Epilepsia. 2011; 52: 1692-1698.
- Phi JH, Paeng JC, Lee HS, Chang Wang K, Kyu Cho B, et al. Evaluation of focal cortical dysplasia and mixed neuronal and glial tumors in pediatric epilepsy patients using 18F-FDG and 11C-methionine pet. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2010; 51: 728-734.
- Maehara T, Nariai T, Arai N, Kawai K, Shimizu H, et al. Usefulness of [11C]methionine PET in the diagnosis of dysembryoplastic neuroepithelial tumor with temporal lobe epilepsy. Epilepsia. 2004; 45: 41-45.
- Didelot A, Mauguiere F, Redoute J, Bouvard S, Lothe A, et al. Voxel-based analysis of asymmetry index maps increases the specificity of 18F-MPPF PET abnormalities for localizing the epileptogenic zone in temporal lobe epilepsies. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2010; 51: 1732-1739.
- Merlet I, Ostrowsky K, Costes N, Ryvlin P, Isnard J, et al. 5-HT1A receptor binding and intracerebral activity in temporal lobe epilepsy: an [18F]MPPF-PET study. Brain: a journal of neurology. 2004; 127: 900-913.
- Assem-Hilger E, Lanzenberger R, Savli M, Wadsak W, Mitterhauser M, et al. Central serotonin 1A receptor binding in temporal lobe epilepsy: a [carbonyl-(11)C]WAY-100635 PET study. Epilepsy & behavior: E&B. 2010; 19: 467-473.
- Meschaks A, Lindstrom P, Halldin C, Farde L, Savic I. Regional reductions in serotonin 1A receptor binding in juvenile myoclonic epilepsy. Archives of neurology. 2005; 62: 946-950.
- Filakovszky J, Gerber K, Bagdy G. A serotonin-1A receptor agonist and an N-methyl-D-aspartate receptor antagonist oppose each others effects in a genetic rat epilepsy model. Neuroscience letters. 1999; 261: 89-92.
- Merlet I, Ryvlin P, Costes N, Dufournel D, Isnard J, et al. Statistical parametric mapping of 5-HT1A receptor binding in temporal lobe epilepsy with hippocampal ictal onset on intracranial EEG. NeuroImage. 2004; 22: 886-896.
- Savic I, Lindstrom P, Gulyas B, Halldin C, Andree B, et al. Limbic reductions of 5-HT1A receptor binding in human temporal lobe epilepsy. Neurology. 2004; 62: 1343-1351.
- Toczek MT, Carson RE, Lang L, Ma Y, Spanaki MV, et al. PET imaging of 5-HT1A receptor binding in patients with temporal lobe epilepsy. Neurology. 2003; 60: 749-756.
- Didelot A, Ryvlin P, Lothe A, Merlet I, Hammers A, et al. PET imaging of brain 5-HT1A receptors in the preoperative evaluation of temporal lobe epilepsy. Brain: a journal of neurology. 2008; 131: 2751-2764.
- Lin TW, de Aburto MA, Dahlbom M, Huang LL, Marvi MM, et al. Predicting seizure-free status for temporal lobe epilepsy patients undergoing surgery: prognostic value of quantifying maximal metabolic asymmetry extending over a specified proportion of the temporal lobe. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2007; 48: 776-782.
- Muzik O, Chugani DC, Shen C, da Silva EA, Shah J, et al. Objective method for localization of cortical asymmetries using positron emission tomography to aid surgical resection of epileptic foci. Computer aided surgery: official journal of the International Society for Computer Aided Surgery. 1998; 3: 74-82.
- Willmann O, Wennberg R, May T, Woermann FG, Pohlmann-Eden B. The contribution of 18F-FDG PET in preoperative epilepsy surgery evaluation for patients with temporal lobe epilepsy A meta-analysis. Seizure: the journal of the British Epilepsy Association. 2007; 16: 509-520.
- Kumar A, Juhasz C, Asano E, Sood S, Muzik O, et al. Objective detection of epileptic foci by 18F-FDG PET in children undergoing epilepsy surgery. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2010; 51: 1901-1907.
- Duncan JS. Imaging in the surgical treatment of epilepsy. Nature reviews. Neurology. 2010; 6: 537-550.
- Salamon N, Kung J, Shaw SJ, Koo J, Koh S, et al. FDG-PET/MRI coregistration improves detection of cortical dysplasia in patients with epilepsy. Neurology. 2008; 71: 1594-1601.
- Lee KK, Salamon N. [18F] fluorodeoxyglucose-positron-emission tomography and MR imaging coregistration for presurgical evaluation of medically refractory epilepsy. AJNR. American journal of neuroradiology. 2009; 30: 1811-1816.
- O’Brien TJ, Miles K, Ware R, Cook MJ, Binns DS, et al. The cost-effective use of 18F-FDG PET in the presurgical evaluation of medically refractory focal epilepsy. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2008; 49: 931-937.
- Vinton AB, Carne R, Hicks RJ, Desmond PM, Kilpatrick C, et al. The extent of resection of FDG-PET hypometabolism relates to outcome of temporal lobectomy. Brain: a journal of neurology. 2007; 130: 548-560.
- Wakamoto H, Chugani DC, Juhasz C, Muzik O, Kupsky WJ, et al. Alpha-methyl-l-tryptophan positron emission tomography in epilepsy with cortical developmental malformations. Pediatric neurology. 2008; 39: 181-188.
- Chassoux F, Rodrigo S, Semah F, Beuvon F, Landre E, et al. FDG-PET improves surgical outcome in negative MRI Taylor-type focal cortical dysplasias. Neurology. 2010; 75: 2168-2175.
- Goffin K, Dedeurwaerdere S, Van Laere K, Van Paesschen W. Neuronuclear assessment of patients with epilepsy. Seminars in nuclear medicine. 2008; 38: 227-239.
- Mazzuca M, Jambaque I, Hertz-Pannier L, Bouilleret V, Archambaud F, et al. 18F-FDG PET reveals frontotemporal dysfunction in children with fever-induced refractory epileptic encephalopathy. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2011; 52: 40-47.
- Akimura T, Yeh HS, Mantil JC, Privitera MD, Gartner M, et al. Cerebral metabolism of the remote area after epilepsy surgery. Neurologia medico-chirurgica. 1999; 39: 16-25.
- Joo EY, Hong SB, Han HJ, Tae WK, Kim JH, et al. Postoperative alteration of cerebral glucose metabolism in mesial temporal lobe epilepsy. Brain: a journal of neurology. 2005; 128: 1802-1810.
- Spanaki MV, Kopylev L, DeCarli C, Gaillard WD, Liow K, et al. Postoperative changes in cerebral metabolism in temporal lobe epilepsy. Archives of neurology. 2000; 57: 1447-1452.
- Muzik O, da Silva EA, Juhasz C, Chugani DC, Shah J, et al. Intracranial EEG versus flumazenil and glucose PET in children with extratemporal lobe epilepsy. Neurology. 2000; 54: 171-179.
- Rosenow F, Luders H. Presurgical evaluation of epilepsy. Brain. 2001; 124: 1683-700.